Abstract
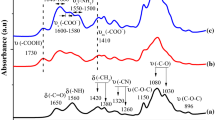
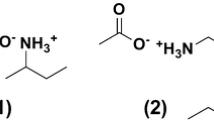
The purpose of this study is to evaluate the newly prepared modified chitosan, a new environmentally friendly adsorbent, in the field of wastewater treatment. Chitosan (CS) reacted with 3-chloro-2,4-pentanedione to give CS derivatives, CS-CPD. Modified CS with O–O and N–O chelating centres was treated with aqueous solution containing different metal ions to investigate its metal uptake and selectivity. The concentration of metal ions in aqueous solution was measured by inductively coupled plasma-optical emission spectrometry. The structure of the complex was identified by elemental analysis, infrared and solid-nuclear magnetic resonance. In addition, the chelating centres were determined by X-ray photoelectron spectroscopy. The morphology of the modified polymer and its metal complexes was studied to show a dramatic change in cases of the CS-CPD–Pb, CS-CPD–Hg, CS-CPD–Cr and CS-CPD–Co complexes.













Similar content being viewed by others
References
Chen B, Zhao H, Chen S, Long F, Huang B, Yang B et al 2019 Chem. Eng. J. (Amsterdam, Neth.) 356 69
Molnar A 2019 Coord. Chem. Rev. 388 126
Ye C-C, An Q-F, Wu J-K, Zhao F-Y, Zheng P-Y and Wang N-X 2019 Chem. Eng. J. (Amsterdam, Neth.) 359 994
Ma C, Li F, Wang C, He M, Shen C, Sand W et al 2018 Environ. Chem. 15 267
Liu W, Qin Y, Liu S, Xing R, Yu H, Chen X et al 2018 Sci. Rep. 8 1
Lebedeva N S, Gubarev Y A, Yurina E S, Vyugin A I and Lipatova I M 2017 Russian J. Gen. Chem. 87 2327
Pestov A and Bratskaya S 2016 Molecules 21 330
Cheung R C F, Ng T B, Wong J H and Chan W Y 2015 Mar. Mar Drugs 13 5156
Li B, Fang Y, Shan C L, Ibrahim M, Xie G L, Wang Y L et al 2013 Asian J. Chem. 25 891
Hadi A G 2012 Br. J. Sci. 5 33
Mekahlia S and Bouzid B 2009 Physics procedia (Proceedings of the JMSM 2008 Conference) 2 1045
Badawy M E I and El-Aswad A F 2012 Plant Protect. Sci. 48 131
Wang X, Du Y and Liu H 2004 Carbohydr. Polym. 56 21
Issam S, Adele M-G, Adele C-P, Stephane G and Veronique C 2005 J. Food Sci. 70 S102
Higazy A, Hashem M, Elshafei A, Shaker N and Abdel Hady M 2010 Carbohydr. Polym. 79 867
Wang X, Du Y, Fan L, Liu V and Hu Y 2005 Polym. Bull. 55 105
Kyoon H, Park N Y, Lee S H and Meyers S P 2002 Int. J. Food Microbiol. 74 65
Sy Q, Daj W and Forster Cf 1998 Water SA 24 251
Ahmad A, Ghufran R and Faizal W M 2010 Clean—Soil, Air, Water 38 153
Zhang L, Zeng Y and Cheng Z 2016 J. Mol. Liq. 214 175
Fu F and Wang Q 2011 J. Environ. Manage. 92 407
Iyengar U and Avs P R 1990 J. Appl. Polym. Sci. 39 739
Findon A, Mckay G and Blair H S 1993 J. Environ. Sci. Health: Part A 28 173
Schmuhl R, Krieg H M and Keizer K 2001 Water SA 27 1
Jung In U and Lone S 2019 Repub. Korean Kong kae Taeho Kongbo, KR 2019027661 A 20190315
Barbosa H F G and Cavalheiro E T G 2019 Int. J. Biol. Macromol. 121 1179
Sandhya B and Tonni A K 2003 J. Hazard. Mater. B97 219
Elassar A-Z A, Al-Fulaij O A and El-Sayed A E M 2010 J. Polym. Res. 17 447
Elassar A-Z A, El-Dissouky A, Jeragh B, Buolian A H and Rizk S 2010 J. Chem. Eng. Data 55 4830
Comans RN J, Eighmy T T and Shaw E L 1996 Surf. Sci. Spectra 4 150
Dalai M K, Kundu R, Pal P, Bhanja M, Sekhar B R and Martin C 2011 J. Alloys Compd. 509 7674
Jiang Y, Lu Y, Wang X, Bao Y, Chen W and Niu L 2014 Nanoscale 6 15066
El-Dissouky A, Elassar A-Z A and Abdul-Hadi B-O 2011 J. Chem. Eng. Data 56 1827
Acknowledgements
We kindly acknowledge the financial support on this project from the Research Administration of the Kuwait University through research project grant SC 12/15. The analytical services provided by the ANALAB and SAF in the faculty of Science through the grant nos. GS-01/01, GS 01/05 and GS 03/08 are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Fulaij, O., Elassar, AZ.A. & Alsagheer, F. Utility of newly modified chitosan in the removal of heavy metal ions from aqueous medium: ion selectivity, XPS and TGA. Bull Mater Sci 42, 237 (2019). https://doi.org/10.1007/s12034-019-1925-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-019-1925-y