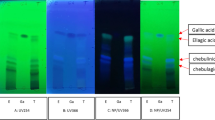
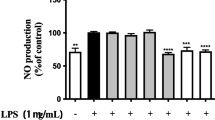
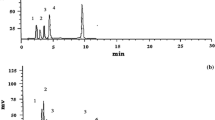
Abstract
Peganum harmala has been extensively employed in Algerian traditional medicine practices. This study aimed to explore the impact of n-butanol (n-BuOH) extract sourced from Peganum harmala seeds on cell proliferation, cell migration, and angiogenesis inhibition. Cytotoxic potential of n-BuOH extract was evaluated using MTT (3-(4,5-dimethylthiazol-2-yl) 2,5 diphenyltetrazolium bromide) assay against human breast adenocarcinoma MCF-7 cells, cell migration was determined using scratch assay, and anti-angiogenic effect was evaluated through macroscopic and histological examinations conducted on chick embryo chorioallantoic membrane. Additionally, this research estimated the phytochemical profile of n-BuOH extract. Fifteen phenolic compounds were identified using Ultra-performance liquid chromatography UPLC-ESI-MS-MS analysis. In addition, the n-BuOH extract of P. harmala exhibited potent antioxidant and free radical scavenging properties. The n-BuOH extract showed potent cytotoxicity against MCF-7 cell with an IC50 value of 8.68 ± 1.58 μg/mL. Furthermore, n-BuOH extract significantly reduced migration. A strong anti-angiogenic activity was observed in the groups treated with n-BuOH extract in comparison to the negative control. Histological analysis confirmed the anti-angiogenic effect of the n-BuOH extract. This activity is probably a result of the synergistic effects produced by different polyphenolic classes.







Similar content being viewed by others
Data availability
Data and material are contained within the article.
References
Al-Ostoot FH, Salah S, Khamees HA, Khanum SA. Tumor angiogenesis: current challenges and therapeutic opportunities. Cancer Treat Res Commun. 2021;28:100422. https://doi.org/10.1016/j.ctarc.2021.100422.
Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–70. https://doi.org/10.1007/s00018-019-03351-7.
Tejerina-Miranda S, Pedrero M, Blázquez-García M, Serafín V, Montero-Calle A, Garranzo-Asensio M, et al. Angiogenesis inhibitor or aggressiveness marker? The function of endostatin in cancer through electrochemical biosensing. Bioelectrochemistry. 2024;155: 108571. https://doi.org/10.1016/j.bioelechem.2023.108571.
Yuan Y, Yuan R, Xin Q, Miao Y, Chen Y, Gao R, et al. Tetramethylpyrazine and paeoniflorin combination (TMP-PF) inhibits angiogenesis in atherosclerosis via miR-126/VEGF/VEGFR2 signaling pathway. J Future Foods. 2024;4:280–7. https://doi.org/10.1016/j.jfutfo.2023.07.010.
Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005. https://doi.org/10.1159/000088478.
Yuan R, Shi W, Xin Q, Yang B, Hoi MP, Lee SM, et al. Tetramethylpyrazine and paeoniflorin inhibit oxidized ldl-induced angiogenesis in human umbilical vein endothelial cells via VEGF and Notch pathways. Evid Based Complement Alternat Med. 2018;2018:e3082507. https://doi.org/10.1155/2018/3082507.
Yang J. The role of reactive oxygen species in angiogenesis and preventing tissue injury after brain ischemia. Microvasc Res. 2019;123:62–7. https://doi.org/10.1016/j.mvr.2018.12.005.
Ghalehbandi S, Yuzugulen J, Pranjol MZI, Pourgholami MH. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur J Pharmacol. 2023;949:175586. https://doi.org/10.1016/j.ejphar.2023.175586.
Kozak J, Jonak K. Association between the antioxidant properties of SESN proteins and anti-cancer therapies. Amino Acids. 2023;55:835–51. https://doi.org/10.1007/s00726-023-03281-6.
Roy A, Khan A, Ahmad I, Alghamdi S, Rajab BS, Babalghith AO, et al. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res Int. 2022;2022:5445291. https://doi.org/10.1155/2022/5445291.
Dehiri M, Diafat A, Fatmi W, Bouaziz F, Khalil R, Bahloul A. Toxicity evaluation of algerian Peganum harmala seed hydromethanolic extract. Toxicol Environ Health Sci. 2022;14:351–9. https://doi.org/10.1007/s13530-022-00149-2.
Bournine L, Bensalem S, Fatmi S, Bedjou F, Mathieu V, Iguer-Ouada M, et al. Evaluation of the cytotoxic and cytostatic activities of alkaloid extracts from different parts of Peganum harmala L. (Zygophyllaceae). Eur J Integr Med. 2017;9:91–6. https://doi.org/10.1016/j.eujim.2016.10.002.
Adeel S, Anjum F, Zuber M, Hussaan M, Amin N, Ozomay M. Sustainable extraction of colourant from harmal seeds (Peganum harmala) for dyeing of bio-mordanted wool fabric. Sustainability. 2022;14:12226. https://doi.org/10.3390/su141912226.
Azzazy HME-S, Sawy AM, Abdelnaser A, Meselhy MR, Shoeib T, Fahmy SA. Peganum harmala alkaloids and tannic acid encapsulated in PAMAM dendrimers: improved anticancer activities as compared to doxorubicin. ACS Appl Polym Mater. 2022;4:7228–39.
Gökkaya İ, Renda G, Subaş T, Özgen U. Phytochemical, pharmacological, and toxicological studies on Peganum harmala L.: an overview of the last decade. Clin Exp Health Sci. 2023. https://doi.org/10.33808/clinexphealthsci.1125345.
Rashid S, Sameti M, Alqarni MH, Abdel Bar FM. In vivo investigation of the inhibitory effect of Peganum harmala L. and its major alkaloids on ethylene glycol-induced urolithiasis in rats. J Ethnopharmacol. 2023;300:115752. https://doi.org/10.1016/j.jep.2022.115752.
Saeedeh F, Oryan S, Ahmadi R, Eidi A. Evaluation of chemical components, anti-oxidant properties, and lethal toxicity of alkaloids extracted from espand (Peganum harmala). J Appl Biol Sci. 2022;16:257–65.
Quezel P, Santa S. Nouvelle flore de l’Algerie : et des regions desertiques meridionales | Wageningen University and Research Library catalog. Paris: CNRS; 1962.
Sfaksi N, Bottone A, Masullo M, Bicha S, Piacente S, Benayache S, et al. Phytochemical investigation of Volutaria lippii and evaluation of the antioxidant activity. Nat Prod Res. 2022. https://doi.org/10.1080/14786419.2022.2138873.
Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. https://doi.org/10.5344/ajev.1965.16.3.144.
Bahorun T, Gressier B, Trotin F, Brunet C, Dine T, Luyckx M, et al. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittelforschung. 1996;46:1086–9.
Koleva II, van Beek TA, Linssen JPH, de Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal PCA. 2002;13:8–17. https://doi.org/10.1002/pca.611.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. https://doi.org/10.1016/s0891-5849(98)00315-3.
Oyaizu M. Studies on products of browning reaction. Jpn J Nutr Diet. 1986;44:307–15. https://doi.org/10.5264/eiyogakuzashi.44.307.
Cacan E, Ozmen ZC. Regulation of Fas in response to bortezomib and epirubicin in colorectal cancer cells. J Chemother. 2020;32:193–201. https://doi.org/10.1080/1120009X.2020.1740389.
Gülmez Y, Aydın A, Can İ, Tekin Ş, Cacan E. Cellular toxicity and biological activities of honey bee (Apis mellifera L.) venom. Marmara Pharm J. 2017. https://doi.org/10.12991/marupj.300329.
Smeriglio A, Denaro M, Barreca D, D’Angelo V, Germanò MP, Trombetta D. Polyphenolic profile and biological activities of black carrot crude extract (Daucus carota L. ssp. sativus var. atrorubens Alef.). Fitoterapia. 2018;124:49–57. https://doi.org/10.1016/j.fitote.2017.10.006.
Pratheeshkumar P, Budhraja A, Son YO, Wang X, Zhang Z, Ding S, et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS ONE. 2012;7:e47516. https://doi.org/10.1371/journal.pone.0047516.
Drabkin DL, Austin JH. Spectrophotometric studies: I. Spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit blood. J Biol Chem. 1932;98:719–33.
Sodaeizadeh HHJ, Van Damme P. Role of phenolic compounds release by Peganum harmala L. on germination and growth suppression of Convolvulus arvensis L. Planta Med. 2009. https://doi.org/10.1055/s-0029-1234344.
Elansary HO, Szopa A, Kubica P, Ekiert H, Al-Mana FA, El-Shafei AA. Polyphenols of Frangula alnus and Peganum harmala leaves and associated biological activities. Plants. 2020;9:1086. https://doi.org/10.3390/plants9091086.
Mounira D, Abdelouahab D, Widad F, Amirouche D, Mansour RB, Rebai K, et al. Acute and chronic toxicity, antioxidant (in vitro and in vivo), and cytotoxic effect of Peganum harmala l. hydromethanolic seeds extract safety profile and biological activities of Peganum harmala. Res Sq. 2021. https://doi.org/10.21203/rs.3.rs-982660/v1.
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200. https://doi.org/10.1038/1811199a0.
Mathew S, Abraham TE, Zakaria ZA. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Technol. 2015;52:5790–8. https://doi.org/10.1007/s13197-014-1704-0.
Alam MZ, Alhebsi MSR, Ghnimi S, Kamal-Eldin A. Inability of total antioxidant activity assays to accurately assess the phenolic compounds of date palm fruit (Phoenix dactylifera L.). NFS J. 2021;22:32–40. https://doi.org/10.1016/j.nfs.2021.01.001.
Platzer M, Kiese S, Tybussek T, Herfellner T, Schneider F, Schweiggert-Weisz U, et al. Radical scavenging mechanisms of phenolic compounds: a quantitative structure-property relationship (QSPR) study. Front Nutr. 2022. https://doi.org/10.3389/fnut.2022.882458.
Shi L, Zhao W, Yang Z, Subbiah V, Suleria HAR. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ Sci Pollut Res. 2022;29:81112–29. https://doi.org/10.1007/s11356-022-23337-6.
Dudonné S, Vitrac X, Coutière P, Woillez M, Mérillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57:1768–74. https://doi.org/10.1021/jf803011r.
Sudan R, Bhagat M, Gupta S, Singh J, Koul A. Iron (FeII) chelation, ferric reducing antioxidant power, and immune modulating potential of arisaema jacquemontii (Himalayan cobra lily). BioMed Res Int. 2014;2014:179865. https://doi.org/10.1155/2014/179865.
Ribatti D, Gualandris A, Bastaki M, Vacca A, Iurlaro M, Roncali L, et al. New model for the study of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane: the gelatin sponge/chorioallantoic membrane assay. J Vasc Res. 1997;34:455–63. https://doi.org/10.1159/000159256.
Ribatti D, Nico B, Vacca A, Presta M. The gelatin sponge-chorioallantoic membrane assay. Nat Protoc. 2006;1:85–91. https://doi.org/10.1038/nprot.2006.13.
Ribatti D. Two new applications in the study of angiogenesis the CAM assay: Acellular scaffolds and organoids. Microvasc Res. 2022;140:104304. https://doi.org/10.1016/j.mvr.2021.104304.
Miura K, Koyanagi-Aoi M, Maniwa Y, Aoi T. Chorioallantoic membrane assay revealed the role of TIPARP (2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible poly (ADP-ribose) polymerase) in lung adenocarcinoma-induced angiogenesis. Cancer Cell Int. 2023;23:34. https://doi.org/10.1186/s12935-023-02870-5.
Huang YJ, Nan GX. Oxidative stress-induced angiogenesis. J Clin Neurosci. 2019;63:13–6. https://doi.org/10.1016/j.jocn.2019.02.019.
Emami MH, Sereshki N, Malakoutikhah Z, Dehkordi SAE, Fahim A, Mohammadzadeh S, et al. Nrf2 signaling pathway in trace metal carcinogenesis: a cross-talk between oxidative stress and angiogenesis. Comp Biochem Physiol Part C Toxicol Pharmacol. 2022;254:109266. https://doi.org/10.1016/j.cbpc.2022.109266.
Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature. 2011;475:226–30. https://doi.org/10.1038/nature10169.
Li F, Bai Y, Zhao M, Huang L, Li S, Li X, et al. Quercetin inhibits vascular endothelial growth factor-induced choroidal and retinal angiogenesis in vitro. Ophthalmic Res. 2015;53:109–16. https://doi.org/10.1159/000369824.
Ghafouri-Fard S, Shoorei H, Khanbabapour Sasi A, Taheri M, Ayatollahi SA. The impact of the phytotherapeutic agent quercetin on expression of genes and activity of signaling pathways. Biomed Pharmacother. 2021;141:111847. https://doi.org/10.1016/j.biopha.2021.111847.
Chin HK, Horng CT, Liu YS, Lu CC, Su CY, Chen PS, et al. Kaempferol inhibits angiogenic ability by targeting VEGF receptor-2 and downregulating the PI3K/AKT, MEK and ERK pathways in VEGF-stimulated human umbilical vein endothelial cells. Oncol Rep. 2018;39:2351–7. https://doi.org/10.3892/or.2018.6312.
Gastaldello GH, Cazeloto ACV, Ferreira JC, Rodrigues DM, Bastos JK, Campo VL, et al. Green propolis compounds (baccharin and p-Coumaric Acid) show beneficial effects in mice for melanoma induced by B16f10. Medicines. 2021;8:20. https://doi.org/10.3390/medicines8050020.
Santhosh Kumar YCT. Anti-angiogenic activity of nano naringenin on chick chorioallantoic membrane model. J Pharm Negat Results. 2022. https://doi.org/10.47750/pnr.2022.13.S07.508.
Acknowledgements
Authors are grateful to the General Directorate for Scientific Research and Technological Development (DGRSDT) of the Algerian Ministry of Higher Education and Scientific Research for the financial support.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
HK performed the experiments, analyzed, interpreted the data, and wrote the main manuscript text. LB supervised all the work, designed and co-performed the experiments (phytochemical and CAM experiments), and then revised the manuscript. DB designed and co-performed CAM experiments. SM co-performed MTT and scratch assay. EC evaluated MTT and scratch assay. MS evaluated experiments and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kemel, H., Benguedouar, L., Boudjerda, D. et al. Phytochemical profiling, cytotoxic, anti-migration, and anti-angiogenic potential of phenolic-rich fraction from Peganum harmala: in vitro and in ovo studies. Med Oncol 41, 144 (2024). https://doi.org/10.1007/s12032-024-02396-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02396-4