Abstract
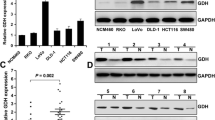
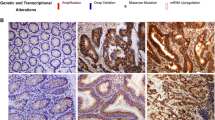
Cancer metastasis remains a major cause of death in cancer patients, and epithelial–mesenchymal transition (EMT) plays a decisive role in cancer metastasis. Recently, abnormal expression of Glycine Decarboxylase (GLDC) has been demonstrated in tumor progression, and GLDC is up-regulated in cancers, such as lung, prostate, bladder, and cervical cancers. However, the exact role of GLDC in colorectal cancer (CRC) progression remains to be elucidated. The aim of our study was to explore the role of GLDC in CRC metastasis. The GSE75117 database was used to investigate GLDC expression in tumor center and invasive front tissues and we found that GLDC expression levels were higher in the invasive front tissue. GLDC expression levels were negatively correlated with the prognosis of CRC patients. In vitro studies have showed that GLDC can promote invasion and migration of CRC cells by inhibiting the Hippo signaling pathway and regulating the EMT process. Blocking the Hippo signaling pathway with Verteporfin reduced the effect of GLDC on CRC metastasis. In vivo metastasis assays further confirmed that tail vein injection of GLDC+/+ cells induced more lung metastasis, compared to normal CRC cells. The results of this study suggest that GLDC promotes EMT through the Hippo signaling pathway, providing a new therapeutic target for CRC metastasis.





Similar content being viewed by others
Data availability
Data will be made available on request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91. https://doi.org/10.1136/gutjnl-2015-310912.
Barugel ME, Vargas C, Krygier WG. Metastatic colorectal cancer: recent advances in its clinical management. Expert Rev Anticancer Ther. 2009;9(12):1829–47. https://doi.org/10.1586/era.09.143.
Wei HY, Feng R, Shao H, Feng B, Liu HQ, Men JL, Zou W. Serum glycine dehydrogenase is associated with increased risk of lung cancer and promotes malignant transformation by regulating DNA methyltransferases expression. Mol Med Rep. 2018;18(2):2293–9. https://doi.org/10.3892/mmr.2018.9214.
Li N, Li H, Cao L, Zhan X. Quantitative analysis of the mitochondrial proteome in human ovarian carcinomas. Endocr Relat Cancer. 2018;25(10):909–31. https://doi.org/10.1530/ERC-18-0243.
Li X, Cui C, Guo Y, Yang G. Glycine decarboxylase expression increased in p53-mutated B cell Lymphoma mice. Oncol Res Treat. 2015;38(11):586–9. https://doi.org/10.1159/000441595.
Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, Bhakoo KK, Jayapal SR, Nichane M, Yu Q, Ahmed DA, Tan C, Sing WP, Tam J, Thirugananam A, Noghabi MS, Pang YH, Ang HS, Mitchell W, Robson P, Kaldis P, Soo RA, Swarup S, Lim EH, Lim B. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148(1–2):259–72. https://doi.org/10.1016/j.cell.2011.11.050.
Go MK, Zhang WC, Lim B, Yew WS. Glycine decarboxylase is an unusual amino acid decarboxylase involved in tumorigenesis. Biochemistry. 2014;53(5):947–56. https://doi.org/10.1021/bi4014227.
Woo CC, Kaur K, Chan WX, Teo XQ, Lee THP. Inhibiting glycine decarboxylase suppresses pyruvate-to-lactate metabolism in lung cancer cells. Front Oncol. 2018;8:196. https://doi.org/10.3389/fonc.2018.00196.
Ma S, Meng Z, Chen R, Guan KL. The hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604. https://doi.org/10.1146/annurev-biochem-013118-111829.
Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–28. https://doi.org/10.1016/j.cell.2015.10.044.
Deng Q, Jiang G, Wu Y, Li J, Liang W, Chen L, Su Q, Li W, Du J, Wong CKC, Chen Z, Wang H. GPER/Hippo-YAP signal is involved in Bisphenol S induced migration of triple negative breast cancer (TNBC) cells. J Hazard Mater. 2018;355:1–9. https://doi.org/10.1016/j.jhazmat.2018.05.013.
Zhao W, Zhang LN, Wang XL, Zhang J, Yu HX. Long noncoding RNA NSCLCAT1 increases non-small cell lung cancer cell invasion and migration through the Hippo signaling pathway by interacting with CDH1. FASEB J. 2019;33(1):1151–66. https://doi.org/10.1096/fj.201800408R.
Ni W, Zhang Y, Zhan Z, Ye F, Liang Y, Huang J, Chen K, Chen L, Ding Y. A novel lncRNA uc. 134 represses hepatocellular carcinoma progression by inhibiting CUL4A-mediated ubiquitination of LATS1. J Hematol Oncol. 2017;10(1):91. https://doi.org/10.1186/s13045-017-0449-4.
Guo PD, Lu XX, Gan WJ, Li XM, He XS, Zhang S, Ji QH, Zhou F, Cao Y, Wang JR, Li JM, Wu H. RARγ downregulation contributes to colorectal tumorigenesis and metastasis by derepressing the hippo-yap pathway. Cancer Res. 2016;76(13):3813–25. https://doi.org/10.1158/0008-5472.CAN-15-2882.
Rawat SJ, Chernoff J. Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem Sci. 2015;40(3):149–56. https://doi.org/10.1016/j.tibs.2015.01.001.
Kittur FS, Lin Y, Arthur E, Hung CY, Li PA, Sane DC, Xie J. Recombinant asialoerythropoetin protects HL-1 cardiomyocytes from injury via suppression of Mst1 activation. Biochem Biophys Rep. 2019;17:157–68. https://doi.org/10.1016/j.bbrep.2019.01.004.
Arthur E, Kittur FS, Lin Y, Hung CY, Sane DC, Xie J. Plant-produced asialo-erythropoietin restores pancreatic beta-cell function by suppressing mammalian sterile-20-like kinase (MST1) and caspase-3 activation. Front Pharmacol. 2017;8:208. https://doi.org/10.3389/fphar.2017.00208.
Guo Z, Li G, Bian E, Ma CC, Wan J, Zhao B. TGF-β-mediated repression of MST1 by DNMT1 promotes glioma malignancy. Biomed Pharmacother. 2017;94:774–80. https://doi.org/10.1016/j.biopha.2017.07.081.
Kuser-Abali G, Alptekin A, Cinar B. Overexpression of MYC and EZH2 cooperates to epigenetically silence MST1 expression. Epigenetics. 2014;9(4):634–43. https://doi.org/10.4161/epi.27957.
Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336(6084):1040–4. https://doi.org/10.1126/science.1218595.
Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, Shelton LM, Gui DY, Kwon M, Ramkissoon SH, Ligon KL, Kang SW, Snuderl M, Vander Heiden MG, Sabatini DM. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520(7547):363–7. https://doi.org/10.1038/nature14363.
Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, Mackay GM, Labuschagne CF, Gay D, Kruiswijk F, Blagih J, Vincent DF, Campbell KJ, Ceteci F, Sansom OJ, Blyth K, Vousden KH. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544(7650):372–6. https://doi.org/10.1038/nature22056.
Nakai T, Nakagawa N, Maoka N, Masui R, Kuramitsu S, Kamiya N. Structure of P-protein of the glycine cleavage system: implications for nonketotic hyperglycinemia. EMBO J. 2005;24(8):1523–36. https://doi.org/10.1038/sj.emboj.7600632.
Kume A, Koyata H, Sakakibara T, Ishiguro Y, Kure S, Hiraga K. The glycine cleavage system. Molecular cloning of the chicken and human glycine decarboxylase cDNAs and some characteristics involved in the deduced protein structures. J Biol Chem. 1991;266(5):3323–9.
Hiraga K, Kikuchi G. The mitochondrial glycine cleavage system. Functional association of glycine decarboxylase and aminomethyl carrier protein. J Biol Chem. 1980;255(24):11671–6.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 82122075; 82074232; 82030118; 82004131; 81830120), Shanghai Frontier Research Base of Disease and Syndrome Biology of Inflammatory Cancer Trans-formation (2021KJ03-12), “Shu Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (21SG43), Three-year Plan Project of Shanghai Traditional Chinese Medicine(ZY(2021–2023)-0208), Clinical Research Plan of SHDC (SHDC2020CR4043), and Shanghai Youth Talent Support Program.
Author information
Authors and Affiliations
Contributions
HY and XH conceived and designed the study. HY and JW analyzed the data. HY and YZ drafted the paper. XH, YW, and HZ revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that there are no conflicts of interest.
Ethical approval
All animal experimental studies have been approved by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine (PZSHUTCM210913009).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, H., Hu, X., Zhang, Y. et al. GLDC promotes colorectal cancer metastasis through epithelial–mesenchymal transition mediated by Hippo signaling pathway. Med Oncol 40, 293 (2023). https://doi.org/10.1007/s12032-023-02076-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02076-9