Abstract
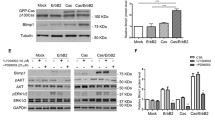
Although Microrchidia 2 (MORC2) is overexpressed in many types of human cancer, its role in breast cancer progression remains unknown. Here, we report that the chromatin remodeler MORC2 expression positively correlates with β-catenin expression in breast cancer cell lines and patients. Overexpression of MORC2 augmented the expression of β-catenin and its target genes, cyclin D1 and c-Myc. Consistent with these results, we found MORC2 knockdown resulted in decreased expression of β-catenin and its target genes. Surprisingly, we observed that c-Myc, the target gene of β-catenin, regulated the MORC2-β-catenin signaling axis through a feedback mechanism. We demonstrated that MORC2 regulates β-catenin expression and function by modulating the phosphorylation of AKT. In addition, we observed reduced proliferation and migration of MORC2 overexpressing breast cancer cells upon β-catenin inhibition. Overall, our results demonstrate that MORC2 promotes breast cancer cell proliferation and migration by regulating β-catenin signaling.






Similar content being viewed by others
Data availability
All the data generated or analyzed during this study is included in this manuscript.
References
Dyba T, Randi G, Bray F, Martos C, Giusti F, Nicholson N, Gavin A, Flego M, Neamtiu L, Dimitrova N, Negrão Carvalho R, Ferlay J, Bettio M. The European cancer burden in 2020: incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 2021;157:308–47.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Riggio AI, Varley KE, Welm AL. The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer. 2020;124:13–26.
Dillekas H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019;8:5574–6.
Xu X, Zhang M, Xu F, Jiang S. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19:165.
Patel S, Alam A, Pant R, Chattopadhyay S. Wnt signaling and its significance within the tumor microenvironment: novel therapeutic insights. Front Immunol. 2020;10:2872.
Chocarro-Calvo A, García-Martínez JM, Ardila-González S, De la Vieja A, García-Jiménez C. Glucose-induced β-catenin acetylation enhances Wnt signaling in cancer. Mol Cell. 2013;49:474–86.
Teulière J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze’ev A, Thiery JP, Glukhova MA. Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005;132:267–77.
Michaelson JS, Leder P. beta-catenin is a downstream effector of Wnt-mediated tumorigenesis in the mammary gland. Oncogene. 2001;20:5093–9.
Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80.
Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–91.
Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51.
Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–9.
Wang H, Zhang L, Luo Q, Liu J, Wang G. MORC protein family-related signature within human disease and cancer. Cell Death Dis. 2021;12:1112.
Wang GL, Wang CY, Cai XZ, Chen W, Wang XH, Li F. Identification and expression analysis of a novel CW-type zinc finger protein MORC2 in cancer cells. Anat Rec (Hoboken). 2010;293:1002–9.
Tong Y, Li Y, Gu H, Wang C, Liu F, Shao Y, Li J, Cao L, Li F. Microchidia protein 2, MORC2, downregulates the cytoskeleton adapter protein, ArgBP2, via histone methylation in gastric cancer cells. Biochem Biophys Res Commun. 2015;467:821–7.
Hong G, Qiu H, Wang C, Jadhav G, Wang H, Tickner J, He W, Xu J. The emerging role of MORC family proteins in cancer development and bone homeostasis. J Cell Physiol. 2016;232:928–34.
Tong Y, Li Y, Gu H, Wang C, Liu F, Shao Y, Li F. HSF1, in association with MORC2, downregulates ArgBP2 via the PRC2 family in gastric cancer cells. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1104–14.
Ding QS, Zhang L, Wang BC, Zeng Z, Zou XQ, Cao PB, Zhou GM, Tang M, Wu L, Wu LL, Yu HG, Guo Y, Zhou FX. Aberrant high expression level of MORC2 is a common character in multiple cancers. Hum Pathol. 2018;76:58–67.
Wang T, Qin ZY, Wen LZ, Guo Y, Liu Q, Lei ZJ, Pan W, Liu KJ, Wang XW, Lai SJ, Sun WJ, Wei YL, Liu L, Guo L, Chen YQ, Wang J, Xiao HL, Bian XW, Chen DF, Wang B. Epigenetic restriction of Hippo signaling by MORC2 underlies stemness of hepatocellular carcinoma cells. Cell Death Differ. 2018;25:2086–100.
Liu M, Sun X, Shi S. MORC2 enhances tumor growth by promoting angiogenesis and tumor-associated macrophage recruitment via Wnt/Î2-catenin in lung cancer. Cell Physiol Biochem. 2018;51:1679–94.
Liao G, Liu X, Wu D, Duan F, Xie X, Wen S, Li Y, Li S. MORC2 promotes cell growth and metastasis in human cholangiocarcinoma and is negatively regulated by miR-186-5p. Aging (Albany NY). 2019;11:3639–49.
Guddeti RK, Chutani N, Pakala SB. MORC2 interactome: its involvement in metabolism and cancer. Biophys Rev. 2021;13:507–14.
Pan Z, Ding Q, Guo Q, Guo Y, Wu L, Tang M, Yu H, Zhou F. MORC2, a novel oncogene, is upregulated in liver cancer and contributes to proliferation, metastasis and chemoresistance. Int J Oncol. 2018;53:59–72.
Liu YY, Liu HY, Yu TJ, Lu Q, Zhang FL, Liu GY, Shao ZM, Li DQ. O-GlcNAcylation of MORC2 at threonine 556 by OGT couples TGF-Î2 signaling to breast cancer progression. Cell Death Differ. 2022;29(4):861–73.
Zhang Q, Song Y, Chen W, Wang X, Miao Z, Cao L, Li F, Wang G. By recruiting HDAC1, MORC2 suppresses p21 Waf1/Cip1 in gastric cancer. Oncotarget. 2015;6:16461–70.
Wang G, Song Y, Liu T, Wang C, Zhang Q, Liu F, Cai X, Miao Z, Xu H, Cao L, Li F. PAK1-mediated MORC2 phosphorylation promotes gastric tumorigenesis. Oncotarget. 2015;6:9877–86.
Liu HY, Liu YY, Yang F, Zhang L, Zhang FL, Hu X, Shao ZM, Li DQ. Acetylation of MORC2 by NAT10 regulates cell-cycle checkpoint control and resistance to DNA-damaging chemotherapy and radiotherapy in breast cancer. Nucleic Acids Res. 2020;48:3638–56.
Xie HY, Zhang TM, Hu SY, Shao ZM, Li DQ. Dimerization of MORC2 through its C-terminal coiled-coil domain enhances chromatin dynamics and promotes DNA repair. Cell Commun Signal. 2019;17:160.
Zhang L, Li DQ. MORC2 regulates DNA damage response through a PARP1-dependent pathway. Nucleic Acids Res. 2019;47:8502–20.
Liao XH, Zhang Y, Dong WJ, Shao ZM, Li DQ. Chromatin remodeling protein MORC2 promotes breast cancer invasion and metastasis through a PRD domain-mediated interaction with CTNND1. Oncotarget. 2017;8:97941–54.
Sánchez-Solana B, Li DQ, Kumar R. Cytosolic functions of MORC2 in lipogenesis and adipogenesis. Biochim Biophys Acta. 2013;1843:316–26.
Guddeti RK, Thomas L, Kannan A, Karyala P, Pakala SB. The chromatin modifier MORC2 affects glucose metabolism by regulating the expression of lactate dehydrogenase A through a feed forward loop with c-Myc. FEBS Lett. 2021;595:1289–302.
Yang F, Xie HY, Yang LF, Zhang L, Zhang FL, Liu HY, Li DQ, Shao ZM. Stabilization of MORC2 by estrogen and antiestrogens through GPER1- PRKACA-CMA pathway contributes to estrogen-induced proliferation and endocrine resistance of breast cancer cells. Autophagy. 2020;16:1061–76.
Albulym OM, Kennerson ML, Harms MB, Drew AP, Siddell AH, Auer-Grumbach M, Pestronk A, Connolly A, Baloh RH, Zuchner S, Reddel SW, Nicholson GA. MORC2 mutations cause axonal Charcot-Marie-Tooth disease with pyramidal signs. Ann Neurol. 2015;79:419–27.
Laššuthová P, Šafka Brožková D, Krůtová M, Mazanec R, Züchner S, Gonzalez MA, Seeman P. Severe axonal Charcot-Marie-Tooth disease with proximal weakness caused by de novo mutation in the MORC2 gene. Brain. 2016;139:e26.
Tchasovnikarova IA, Timms RT, Douse CH, Roberts RC, Dougan G, Kingston RE, Modis Y, Lehner PJ. Hyperactivation of HUSH complex function by Charcot-Marie-Tooth disease mutation in MORC2. Nat Genet. 2017;49:1035–44.
Sancho P, Bartesaghi L, Miossec O, García-García F, Ramírez-Jiménez L, Siddell A, Åkesson E, Hedlund E, Laššuthová P, Pascual-Pascual SI, Sevilla T, Kennerson M, Lupo V, Chrast R, Espinós C. Characterization of molecular mechanisms underlying the axonal Charcot-Marie-Tooth neuropathy caused by MORC2 mutations. Hum Mol Genet. 2019;28:1629–44.
Zhang FL, Cao JL, Xie HY, Sun R, Yang LF, Shao ZM, Li DQ. Cancer-associated MORC2-mutant M276I regulates an hnRNPM-mediated CD44 splicing switch to promote invasion and metastasis in triple-negative breast cancer. Cancer Res. 2018;78:5780–92.
Kan Z, Ding Y, Kim J, Jung HH, Chung W, Lal S, Cho S, Fernandez-Banet J, Lee SK, Kim SW, Lee JE, Choi YL, Deng S, Kim JY, Ahn JS, Sha Y, Mu XJ, Nam JY, Im YH, Lee S, Park WY, Nam SJ, Park YH. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9:1725.
Pozniak Y, Balint-Lahat N, Rudolph JD, Lindskog C, Katzir R, Avivi C, Pontén F, Ruppin E, Barshack I, Geiger T. System-wide clinical proteomics of breast cancer reveals global remodeling of tissue homeostasis. Cell Syst. 2016;2:172–84.
Zhang J, Yang Y, Dong Y, Liu C. Microrchidia family C-type zinc finger 2 promotes the proliferation, invasion, migration and epithelial-mesenchymal transition of glioma by regulating PTEN/PI3K/AKT signaling via binding to N-myc downstream regulated gene 1 promoter. Int J Mol Med. 2021. https://doi.org/10.3892/ijmm.2021.5071.
Su Y, Yu T, Wang Y, Huang X, Wei X. Circular RNA circDNM3OS: functions as a miR-145–5p sponge to accelerate cholangiocarcinoma growth and glutamine metabolism by upregulating MORC2. Onco Targets Ther. 2021;14:1117–29.
Popolo A, Pinto A, Daglia M, Nabavi SF, Farooqi AA, Rastrelli L. Two likely targets for the anti-cancer effect of indole derivatives from cruciferous vegetables: PI3K/Akt/mTOR signalling pathway and the aryl hydrocarbon receptor. Semin Cancer Biol. 2017;46:132–7.
Dai H, Deng HB, Wang YH, Guo JJ. Resveratrol inhibits the growth of gastric cancer via the Wnt/β-catenin pathway. Oncol Lett. 2018;16:1579–83.
Acknowledgements
We are thankful to IISER Tirupati for providing the financial support. RKG is thankful to DBT for awarding SRF and providing the financial support.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
HSS, RKG, JPJ, KKP, PK, and SBP performed experiments and analyzed data. SBP supervised the entire project, and designed the experiments. HSS, RKG, and SBP wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saroha, H.S., Kumar Guddeti, R., Jacob, J.P. et al. MORC2/β-catenin signaling axis promotes proliferation and migration of breast cancer cells. Med Oncol 39, 135 (2022). https://doi.org/10.1007/s12032-022-01728-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01728-6