Abstract
Organ crosstalk is a complex biological communication between distal organs mediated via cellular, soluble, and neurohormonal actions, based on a two-way pathway. The communication between the central nervous system and peripheral organs involves nerves, endocrine, and immunity systems as well as the emotional and cognitive centers of the brain. Particularly, acute brain injury is complicated by neuroinflammation and neurodegeneration causing multiorgan inflammation, microbial dysbiosis, gastrointestinal dysfunction and dysmotility, liver dysfunction, acute kidney injury, and cardiac dysfunction. Organ crosstalk has become increasingly popular, although the information is still limited. The present narrative review provides an update on the crosstalk between the nervous system and systemic organs after acute brain injury. Future research might help to target this pathophysiological process, preventing the progression toward multiorgan dysfunction in critically ill patients with brain injury.



Similar content being viewed by others
Change history
26 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12028-023-01745-x
References
Husain-Syed F, McCullough PA, Birk H-W, Renker M, Brocca A, Seeger W, Ronco C. Cardio-pulmonary-renal interactions. J Am Coll Cardiol. 2015;65:2433–48. https://doi.org/10.1016/j.jacc.2015.04.024.
Battaglini D, da Silva AL, Felix NS, Rodrigues G, Antunes MA, Rocha NN, Capelozzi VL, Morales MM, Cruz FF, Robba C, Silva PL, Pelosi P, Rocco PRM. Mild hypothermia combined with dexmedetomidine reduced brain, lung, and kidney damage in experimental acute focal ischemic stroke. Intensive Care Med Exp. 2022;10:53. https://doi.org/10.1186/s40635-022-00481-4.
Meyfroidt G, Baguley IJ, Menon DK. Paroxysmal sympathetic hyperactivity: the storm after acute brain injury. Lancet Neurol. 2017;16:721–9. https://doi.org/10.1016/S1474-4422(17)30259-4.
Smith M, Meyfroidt G. Focus on the brain and systemic organ systems: when essential interactions become toxic relationships. Intensive Care Med. 2018;44:2263–6. https://doi.org/10.1007/s00134-018-5439-7.
Anthony DC, Couch Y. The systemic response to CNS injury. Exp Neurol. 2014;258:105–11. https://doi.org/10.1016/j.expneurol.2014.03.013.
Saran S, Gurjar M. Brain crosstalk with other organs in ICU patient. J Neuroanaesth Crit Care. 2019;06:299–304. https://doi.org/10.1055/s-0039-3399474.
Eppensteiner J, Lee J. Damage-associated molecular patterns in critical illness and multi-organ failure. J Emerg Crit Care Med. 2018;2:80–80. https://doi.org/10.21037/jeccm.2018.10.04.
Battaglini D, Pimentel-Coelho PM, Robba C, dos Santos CC, Cruz FF, Pelosi P, Rocco PRM. Gut microbiota in acute ischemic stroke: from pathophysiology to therapeutic implications. Front Neurol. 2020;11:598. https://doi.org/10.3389/fneur.2020.00598.
Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2018;28:203–9.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444:1022–3. https://doi.org/10.1038/4441022a.
Holtmann G, Talley NJ. The stomach–brain axis. Best Pract Res Clin Gastroenterol. 2014;28:967–79. https://doi.org/10.1016/j.bpg.2014.10.001.
Thor PJ, Gościński I, Kolasińska-Kloch W, Madroszkiewicz D, Madroszkiewicz E, Furgała A. Gastric myoelectric activity in patients with closed head brain injury. Med Sci Monit. 2003;9:CR392-5.
Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv. 2008;8:242–53. https://doi.org/10.1124/mi.8.5.9.
Holzer P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr Opin Pharmacol. 2007;7:563–9. https://doi.org/10.1016/j.coph.2007.09.004.
Masaoka T, Tack J. Gastroparesis: current concepts and management. Gut Liver. 2009;3:166–73. https://doi.org/10.5009/gnl.2009.3.3.166.
Btaiche IF, Chan L-N, Pleva M, Kraft MD. Critical Illness, gastrointestinal complications, and medication therapy during enteral feeding in critically Ill adult patients. Nutr Clin Pract. 2010;25:32–49. https://doi.org/10.1177/0884533609357565.
Reintam Blaser A, Poeze M, Malbrain MLNG, Björck M, Oudemans-van Straaten HM, Starkopf J. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: a prospective multicentre study. Intensive Care Med. 2013;39:899–909. https://doi.org/10.1007/s00134-013-2831-1.
Reintam Blaser A, Malbrain MLNG, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun J-P, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384–94. https://doi.org/10.1007/s00134-011-2459-y.
Ott L, Young B, Phillips R, McClain C, Adams L, Dempsey R, Tibbs P, Ryo UY. Altered gastric emptying in the head-injured patient: relationship to feeding intolerance. J Neurosurg. 1991;74:738–42. https://doi.org/10.3171/jns.1991.74.5.0738.
Kao C-H, ChangLai S-P, Chieng P-U, Yen T-C. Gastric emptying in head-injured patients. Am J Gastroenterol. 1998;93:1108–12. https://doi.org/10.1111/j.1572-0241.1998.00338.x.
Norton JA, Ott LG, McClain C, Adams L, Dempsey RJ, Haack D, Tibbs PA, Young AB. Intolerance to enteral feeding in the brain-injured patient. J Neurosurg. 1988;68:62–6. https://doi.org/10.3171/jns.1988.68.1.0062.
Jackson MD, Davidoff G. Gastroparesis following traumatic brain injury and response to metoclopramide therapy. Arch Phys Med Rehabil. 1989;70:553–5.
Saxe JM, Ledgerwood AM, Lucas CE, Lucas WF. Lower esophageal sphincter dysfunction precludes safe gastric feeding after head injury. J Trauma Injury Infect Crit Care. 1994;37:581–6. https://doi.org/10.1097/00005373-199410000-00010.
Garrick T, Mulvihill S, Buack S, Maeda-Hagiwara M, Tache Y. Intracerebroventricular pressure inhibits gastric antral and duodenal contractility but not acid secretion in conscious rabbits. Gastroenterology. 1988;95:26–31. https://doi.org/10.1016/0016-5085(88)90286-7.
Asrani VM, Brown A, Huang W, Bissett I, Windsor JA. Gastrointestinal dysfunction in critical Illness: a review of scoring tools. J Parenter Enter Nutr. 2020;44:182–96. https://doi.org/10.1002/jpen.1679.
Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, Fruhwald S, Hiesmayr M, Ichai C, Jakob SM, Loudet CI, Malbrain MLNG, Montejo González JC, Paugam-Burtz C, Poeze M, Preiser J-C, Singer P, van Zanten ARH, De Waele J, Wendon J, Wernerman J, Whitehouse T, Wilmer A, Oudemans-van Straaten HM. Early enteral nutrition in critically Ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43:380–98. https://doi.org/10.1007/s00134-016-4665-0.
Chourdakis M, Kraus MM, Tzellos T, Sardeli C, Peftoulidou M, Vassilakos D, Kouvelas D. Effect of early compared with delayed enteral nutrition on endocrine function in patients with traumatic brain injury. J Parenter Enter Nutr. 2012;36:108–16. https://doi.org/10.1177/0148607110397878.
Chan KH, Mann KS, Lai EC, Ngan J, Tuen H, Yue CP. Factors influencing the development of gastrointestinal complications after neurosurgery. Neurosurgery. 1989. https://doi.org/10.1097/00006123-198909000-00010.
Misra UK, Kalita J, Pandey S, Mandal SK. Predictors of gastrointestinal bleeding in acute intracerebral haemorrhage. J Neurol Sci. 2003;208:25–9. https://doi.org/10.1016/S0022-510X(02)00415-X.
Liu B, Li B, Zhang X, Fei Z, Hu S, Lin W, Gao D, Zhang L. A randomized controlled study comparing omeprazole and cimetidine for the prophylaxis of stress-related upper gastrointestinal bleeding in patients with intracerebral hemorrhage. J Neurosurg. 2013;118:115–20. https://doi.org/10.3171/2012.9.JNS12170.
Wozniak H, Beckmann TS, Fröhlich L, Soccorsi T, Le Terrier C, de Watteville A, Schrenzel J, Heidegger C-P. The central and biodynamic role of gut microbiota in critically Ill patients. Crit Care. 2022;26:250. https://doi.org/10.1186/s13054-022-04127-5.
Malbrain MLNG, De Laet I. AIDS is coming to your ICU: be prepared for acute bowel injury and acute intestinal distress syndrome. Intensive Care Med. 2008;34:1565–9. https://doi.org/10.1007/s00134-008-1135-3.
Perova-Sharonova V, Albokrinov A, Fesenko U, Gutor T. Effect of intraabdominal hypertension on splanchnic blood flow in children with appendicular peritonitis. J Anaesthesiol Clin Pharmacol. 2021;37:360. https://doi.org/10.4103/joacp.JOACP_293_19.
Depauw PRAM, Groen RJM, Van Loon J, Peul WC, Malbrain MLNG, De Waele JJ. The significance of intra-abdominal pressure in neurosurgery and neurological diseases: a narrative review and a conceptual proposal. Acta Neurochir (Wien). 2019;161:855–64. https://doi.org/10.1007/s00701-019-03868-7.
Malbrain MLNG, Peeters Y, Wise R. The neglected role of abdominal compliance in organ-organ interactions. Crit Care. 2016;20:67. https://doi.org/10.1186/s13054-016-1220-x.
Kimball EJ. Intra-abdominal hypertension and abdominal compartment syndrome: a current review. Curr Opin Crit Care. 2021;27:164–8. https://doi.org/10.1097/MCC.0000000000000797.
De Laet IE, Malbrain MLNG, De Waele JJ. A clinician’s guide to management of intra-abdominal hypertension and abdominal compartment syndrome in critically Ill patients. Crit Care. 2020;24:97. https://doi.org/10.1186/s13054-020-2782-1.
Härtl R, Gerber LM, Ni Q, Ghajar J. Effect of early nutrition on deaths due to severe traumatic brain injury. J Neurosurg. 2008;109:50–6. https://doi.org/10.3171/JNS/2008/109/7/0050.
Roberts DJ, Ball CG, Kirkpatrick AW. Increased pressure within the abdominal compartment. Curr Opin Crit Care. 2016;22:1. https://doi.org/10.1097/MCC.0000000000000289.
Leng Y, Yi M, Fan J, Bai Y, Ge Q, Yao G. Effects of acute intra-abdominal hypertension on multiple intestinal barrier functions in rats. Sci Rep. 2016;6:22814. https://doi.org/10.1038/srep22814.
Zanza C, Romenskaya T, Thangathurai D, Ojetti V, Saviano A, Abenavoli L, Robba C, Cammarota G, Franceschi F, Piccioni A, Longhitano Y. Microbiome in critical care: an unconventional and unknown ally. Curr Med Chem. 2022;29:3179–88. https://doi.org/10.2174/0929867328666210915115056.
Martin-Loeches I, Dickson R, Torres A, Hanberger H, Lipman J, Antonelli M, de Pascale G, Bozza F, Vincent JL, Murthy S, Bauer M, Marshall J, Cilloniz C, Bos LD. The importance of airway and lung microbiome in the critically Ill. Crit Care. 2020;24:537. https://doi.org/10.1186/s13054-020-03219-4.
Xu R, Tan C, Zhu J, Zeng X, Gao X, Wu Q, Chen Q, Wang H, Zhou H, He Y, Pan S, Yin J. Dysbiosis of the intestinal microbiota in neurocritically Ill patients and the risk for death. Crit Care. 2019;23:195. https://doi.org/10.1186/s13054-019-2488-4.
Taraskina A, Ignatyeva O, Lisovaya D, Ivanov M, Ivanova L, Golovicheva V, Baydakova G, Silachev D, Popkov V, Ivanets T, Kashtanova D, Yudin V, Makarov V, Abramov I, Lukashina M, Rakova V, Zagainova A, Zorov D, Plotnikov E, Sukhikh G, Yudin S. Effects of traumatic brain injury on the gut microbiota composition and serum amino acid profile in rats. Cells. 2022. https://doi.org/10.3390/cells11091409.
Maltsev AV, Bystryak S, Galzitskaya OV. The role of β-amyloid peptide in neurodegenerative diseases. Ageing Res Rev. 2011;10:440–52. https://doi.org/10.1016/j.arr.2011.03.002.
Goldsworthy MR, Vallence A-M. The role of -amyloid in Alzheimer’s disease-related neurodegeneration. J Neurosci. 2013;33:12910–1. https://doi.org/10.1523/JNEUROSCI.2252-13.2013.
Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–3. https://doi.org/10.1182/blood-2005-06-2506.
Zou C-G, Zhao Y-S, Gao S-Y, Li S-D, Cao X-Z, Zhang M, Zhang K-Q. Homocysteine promotes proliferation and activation of microglia. Neurobiol Aging. 2010;31:2069–79. https://doi.org/10.1016/j.neurobiolaging.2008.11.007.
Zinno P, Motta V, Guantario B, Natella F, Roselli M, Bello C, Comitato R, Carminati D, Tidona F, Meucci A, Aiello P, Perozzi G, Virgili F, Trevisi P, Canali R, Devirgiliis C. Supplementation with dairy matrices impacts on homocysteine levels and gut microbiota composition of hyperhomocysteinemic mice. Eur J Nutr. 2020;59:345–58. https://doi.org/10.1007/s00394-019-01911-y.
Freedberg DE, Zhou MJ, Cohen ME, Annavajhala MK, Khan S, Moscoso DI, Brooks C, Whittier S, Chong DH, Uhlemann A-C, Abrams JA. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 2018;44:1203–11. https://doi.org/10.1007/s00134-018-5268-8.
Ojima M, Shimizu K, Motooka D, Ishihara T, Nakamura S, Shintani A, Ogura H, Iida T, Yoshiya K, Shimazu T. Gut dysbiosis associated with antibiotics and disease severity and its relation to mortality in critically Ill patients. Dig Dis Sci. 2022;67:2420–32. https://doi.org/10.1007/s10620-021-07000-7.
Taraskina A, Ignatyeva O, Lisovaya D, Ivanov M, Ivanova L, Golovicheva V, Baydakova G, Silachev D, Popkov V, Ivanets T, Kashtanova D, Yudin V, Makarov V, Abramov I, Lukashina M, Rakova V, Zagainova A, Zorov D, Plotnikov E, Sukhikh G, Yudin S. Effects of traumatic brain injury on the gut microbiota composition and serum amino acid profile in rats. Cells. 2022;11:1409. https://doi.org/10.3390/cells11091409.
Celorrio Navarro M, Shumilov K, Rodgers R, Schriefer L, Li Y, Baldridge MT, Friess S. Innate and peripheral immune alterations after TBI are regulated in a gut microbiota-dependent manner in mice. J Neurotrauma. 2022. https://doi.org/10.1089/neu.2022.0356.
Howard BM, Kornblith LZ, Christie SA, Conroy AS, Nelson MF, Campion EM, Callcut RA, Calfee CS, Lamere BJ, Fadrosh DW, Lynch S, Cohen MJ. Characterizing the gut microbiome in trauma: significant changes in microbial diversity occur early after severe injury. Trauma Surg Acute Care Open. 2017;2:e000108. https://doi.org/10.1136/tsaco-2017-000108.
Matzaras R, Nikopoulou A, Protonotariou E, Christaki E. Gut microbiota modulation and prevention of dysbiosis as an alternative approach to antimicrobial resistance: a narrative review. Yale J Biol Med. 2022;95:479–94.
van Prehn J, Reigadas E, Vogelzang EH, Bouza E, Hristea A, Guery B, Krutova M, Norén T, Allerberger F, Coia JE, Goorhuis A, van Rossen TM, Ooijevaar RE, Burns K, Scharvik Olesen BR, Tschudin-Sutter S, Wilcox MH, Vehreschild MJGT, Fitzpatrick F, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. 2021;27:S1–21. https://doi.org/10.1016/j.cmi.2021.09.038.
Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016;20:262. https://doi.org/10.1186/s13054-016-1434-y.
Wang Y, Ye Z, Ge L, Siemieniuk RAC, Wang X, Wang Y, Hou L, Ma Z, Agoritsas T, Vandvik PO, Perner A, Møller MH, Guyatt GH, Liu L. Efficacy and safety of gastrointestinal bleeding prophylaxis in critically Ill patients: systematic review and network meta-analysis. BMJ. 2020. https://doi.org/10.1136/bmj.l6744.
Lelubre C, Bouzat P, Crippa IA, Taccone FS. Anemia management after acute brain injury. Crit Care. 2016;20:152. https://doi.org/10.1186/s13054-016-1321-6.
Cooper AS. Interventions for preventing upper gastrointestinal bleeding in people admitted to intensive care units. Crit Care Nurse. 2019;39:102–3. https://doi.org/10.4037/ccn2019916.
Young PJ, Bagshaw SM, Forbes AB, Nichol AD, Wright SE, Bailey M, Bellomo R, Beasley R, Brickell K, Eastwood GM, Gattas DJ, van Haren F, Litton E, Mackle DM, McArthur CJ, McGuinness SP, Mouncey PR, Navarra L, Opgenorth D, Pilcher D, Saxena MK, Webb SA, Wiley D, Rowan KM. Effect of stress ulcer prophylaxis with proton pump inhibitors vs histamine-2 receptor blockers on in-hospital mortality among ICU patients receiving invasive mechanical ventilation. JAMA. 2020;323:616. https://doi.org/10.1001/jama.2019.22190.
Krag M, Marker S, Perner A, Wetterslev J, Wise MP, Schefold JC, Keus F, Guttormsen AB, Bendel S, Borthwick M, Lange T, Rasmussen BS, Siegemund M, Bundgaard H, Elkmann T, Jensen JV, Nielsen RD, Liboriussen L, Bestle MH, Elkjær JM, Palmqvist DF, Bäcklund M, Laake JH, Bådstøløkken PM, Grönlund J, Breum O, Walli A, Winding R, Iversen S, Jarnvig I-L, White JO, Brand B, Madsen MB, Quist L, Thornberg KJ, Møller A, Wiis J, Granholm A, Anthon CT, Meyhoff TS, Hjortrup PB, Aagaard SR, Andreasen JB, Sørensen CA, Haure P, Hauge J, Hollinger A, Scheuzger J, Tuchscherer D, Vuilliomenet T, Takala J, Jakob SM, Vang ML, Pælestik KB, Andersen KLD, van der Horst ICC, Dieperink W, Fjølner J, Kjer CKW, Sølling C, Sølling CG, Karttunen J, Morgan MPG, Sjøbø B, Engstrøm J, Agerholm-Larsen B, Møller MH. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med. 2018;379:2199–208. https://doi.org/10.1056/NEJMoa1714919.
Hammond DA, Kathe N, Shah A, Martin BC. Cost-effectiveness of histamine 2 receptor antagonists versus proton pump inhibitors for stress ulcer prophylaxis in critically Ill patients. Pharmacother J Hum Pharmacol Drug Ther. 2017;37:43–53. https://doi.org/10.1002/phar.1859.
Lin P-C, Chang C-H, Hsu P-I, Tseng P-L, Huang Y-B. The efficacy and safety of proton pump inhibitors vs histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: a meta-analysis. Crit Care Med. 2010;38:1197–205. https://doi.org/10.1097/CCM.0b013e3181d69ccf.
Matsubara Y, Kiyohara H, Teratani T, Mikami Y, Kanai T. Organ and brain crosstalk: the liver-brain axis in gastrointestinal, liver, and pancreatic diseases. Neuropharmacology. 2022;205:108915. https://doi.org/10.1016/j.neuropharm.2021.108915.
Villapol S. Consequences of hepatic damage after traumatic brain injury: current outlook and potential therapeutic targets. Neural Regen Res. 2016;11:226. https://doi.org/10.4103/1673-5374.177720.
De Buck M, Gouwy M, Wang JM, Van Snick J, Proost P, Struyf S, Van Damme J. The cytokine-serum amyloid A-chemokine network. Cytokine Growth Factor Rev. 2016;30:55–69. https://doi.org/10.1016/j.cytogfr.2015.12.010.
Theytaz F, de Giorgi S, Hodson L, Stefanoni N, Rey V, Schneiter P, Giusti V, Tappy L. Metabolic fate of fructose ingested with and without glucose in a mixed meal. Nutrients. 2014;6:2632–49. https://doi.org/10.3390/nu6072632.
Filipović B, Marković O, Đurić V, Filipović B. Cognitive changes and brain volume reduction in patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol. 2018;2018:1–6. https://doi.org/10.1155/2018/9638797.
Seo SW, Gottesman RF, Clark JM, Hernaez R, Chang Y, Kim C, Ha KH, Guallar E, Lazo M. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology. 2016;86:1136–42. https://doi.org/10.1212/WNL.0000000000002498.
Rege SD, Royes L, Tsai B, Zhang G, Yang X, Gomez-Pinilla F. Brain trauma disrupts hepatic lipid metabolism: blame it on fructose? Mol Nutr Food Res. 2019;63:e1801054. https://doi.org/10.1002/mnfr.201801054.
Moore EM, Bellomo R, Nichol A, Harley N, Macisaac C, Cooper DJ. The incidence of acute kidney injury in patients with traumatic brain injury. Ren Fail. 2010;32:1060–5. https://doi.org/10.3109/0886022X.2010.510234.
Park C-Y, Choi H-Y, You N-K, Roh TH, Seo SJ, Kim S-H. Continuous renal replacement therapy for acute renal failure in patients with traumatic brain injury. Korean J Neurotrauma. 2016;12:89. https://doi.org/10.13004/kjnt.2016.12.2.89.
Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical Renin-Angiotensin system in kidney physiology. Compr Physiol. 2014;4:1201–28. https://doi.org/10.1002/cphy.c130040.
Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–70. https://doi.org/10.1681/ASN.2007080901.
Cao W, Li A, Li J, Wu C, Cui S, Zhou Z, Liu Y, Wilcox CS, Hou FF. Reno-cerebral reflex activates the Renin-Angiotensin system, promoting oxidative stress and renal damage after ischemia-reperfusion injury. Antioxid Redox Signal. 2017;27:415–32. https://doi.org/10.1089/ars.2016.6827.
Nongnuch A, Panorchan K, Davenport A. Brain-kidney crosstalk. Crit Care. 2014;18:225. https://doi.org/10.1186/cc13907.
Yang B, Xu J, Xu F, Zou Z, Ye C, Mei C, Mao Z. Intravascular administration of mannitol for acute kidney injury prevention: a systematic review and meta-analysis. PLoS One. 2014;9:e85029. https://doi.org/10.1371/journal.pone.0085029.
Pesonen A, Ben-Hamouda N, Schneider A. Acute kidney injury after brain injury: does it exist? Minerva Anestesiol. 2021;87:823–7. https://doi.org/10.23736/S0375-9393.20.14991-5.
Khalid F, Yang GL, McGuire JL, Robson MJ, Foreman B, Ngwenya LB, Lorenz JN. Autonomic dysfunction following traumatic brain injury: translational insights. Neurosurg Focus. 2019;47:E8. https://doi.org/10.3171/2019.8.FOCUS19517.
Edwards BS, Zimmerman RS, Schwab TR, Heublein DM, Burnett JC. Atrial stretch, not pressure, is the principal determinant controlling the acute release of atrial natriuretic factor. Circ Res. 1988;62:191–5. https://doi.org/10.1161/01.RES.62.2.191.
Marin-Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature. 1986;324:473–6. https://doi.org/10.1038/324473a0.
Udy A, Boots R, Senthuran S, Stuart J, Deans R, Lassig-Smith M, Lipman J. Augmented renal clearance in traumatic brain injury. Crit Care. 2010;14:P521. https://doi.org/10.1186/cc8753.
Udy AA, Jarrett P, Lassig-Smith M, Stuart J, Starr T, Dunlop R, Deans R, Roberts JA, Senthuran S, Boots R, Bisht K, Bulmer AC, Lipman J. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide, cardiac output, and creatinine clearance. J Neurotrauma. 2017;34:137–44. https://doi.org/10.1089/neu.2015.4328.
Dickerson RN, Crawford CN, Tsiu MK, Bujanowski CE, van Matre ET, Swanson JM, Filiberto DM, Minard G. Augmented renal clearance following traumatic injury in critically Ill patients requiring nutrition therapy. Nutrients. 2021;13:5110. https://doi.org/10.3390/nu13051681.
Ghoshal S, Yang V, Brodie D, Radhakrishnan J, Roh DJ, Park S, Claassen J, Agarwal S. In-hospital survival and neurological recovery among patients requiring renal replacement therapy in post-cardiac arrest period. Kidney Int Rep. 2019;4:674–8. https://doi.org/10.1016/j.ekir.2019.02.004.
Davenport A. Continuous renal replacement therapies in patients with acute neurological injury. Semin Dial. 2009;22:165–8. https://doi.org/10.1111/j.1525-139X.2008.00548.x.
Robba C, Rebora P, Banzato E, Wiegers EJA, Stocchetti N, Menon DK, Citerio G, Åkerlund C, Nelson D, Amrein K, Nyirádi J, Andelic N, Andreassen L, Anke A, Audibert G, Azouvi P, Azzolini ML, Beretta L, Calvi MR, Bartels R, den Boogert H, Beer R, Helbok R, Bellander B-M, Benali H, Degos V, Galanaud D, Perlbarg V, Vanhaudenhuyse A, Berardino M, Blaabjerg M, Lund SB, Brorsson C, Buki A, Czeiter E, Cabeleira M, Czosnyka M, Smielewski P, Caccioppola A, Calappi E, Carbonara M, Mulazzi D, Ortolano F, Zoerle T, Cameron P, Gantner D, Murray L, Trapani T, Vallance S, Lozano GC, Pomposo I, Castaño-León AM, Gomez PA, Lagares A, Chevallard G, Chieregato A, Citerio G, Coburn M, Kowark A, Rossaint R, Coles J, Cooper JD, Correia M, Dahyot-Fizelier C, De Keyser V, Maas AIR, Menovsky T, Nair N, Van der Steen G, Della Corte F, Grossi F, Depreitere B, Dilvesi D, Golubovic J, Karan M, Vulekovic P, Dixit A, Ercole A, Koraropoulos E, Menon D, Newcombe V, Richter S, Stamatakis E, Williams G, Winzeck S, Zeiler FA, Dreier J, Dulière G-L, Maréchal H, Ezer E, Vámos Z, Fabricius M, Kondziella D, Foks K, Frisvold S, Furmanov A, Rosenthal G, Ghuysen A, Giga L, Valeinis E, Ziverte A, Gupta D, Haitsma I, Volovici V, Helseth E, Hutchinson PJ, Kolias AG, Jankowski S, Koskinen L-O, Kovács N, Laureys S, Noirhomme Q, Vanhaudenhuyse A, Lejeune A, Vega E, Lightfoot R, Steyerberg EW, Lingsma H, Voormolen D, Manara A, Thomas M, Martino C, Mattern J, Sakowitz O, Younsi A, McMahon C, Muraleedharan V, Negru A, Ples H, Tudora CM, Payen J-F, Persona P, Rossi S, Peul W, van Dijck JTJM, van Essen TA, van Wijk RPJ, Piippo-Karjalainen A, Raj R, Posti JP, Tenovuo O, Puybasset L, Radoi A, Sahuquillo J, Ragauskas A, Rocka S, Rhodes J, Roe C, Roise O, Rosenfeld JV, Rosenlund C, Sandro O, Schirmer-Mikalsen K, Sakowitz O, Sanchez-Porras R, Schirmer-Mikalsen K, Vik A, Schou RF, Sorinola A, Tamás V, Steyerberg EW, Stocchetti N, Sundström N, Takala R, Tamosuitis T, Tibboel D, Tolias C, Vajkoczy P, Vargiolu A, Vik A, Vilcinis R, Wolf S, Zeiler FA. Incidence, risk factors, and effects on outcome of ventilator-associated pneumonia in patients with traumatic brain injury. Chest. 2020;158:2292–303. https://doi.org/10.1016/j.chest.2020.06.064.
Borsellino B, Schultz MJ, Gama de Abreu M, Robba C, Bilotta F. Mechanical ventilation in neurocritical care patients: a systematic literature review. Expert Rev Respir Med. 2016;10:1123–32. https://doi.org/10.1080/17476348.2017.1235976.
Robba C, Poole D, McNett M, Asehnoune K, Bösel J, Bruder N, Chieregato A, Cinotti R, Duranteau J, Einav S, Ercole A, Ferguson N, Guerin C, Siempos II, Kurtz P, Juffermans NP, Mancebo J, Mascia L, McCredie V, Nin N, Oddo M, Pelosi P, Rabinstein AA, Neto AS, Seder DB, Skrifvars MB, Suarez JI, Taccone FS, van der Jagt M, Citerio G, Stevens RD. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med. 2020;46:2397–410. https://doi.org/10.1007/s00134-020-06283-0.
Robba C, Ball L, Nogas S, Battaglini D, Messina A, Brunetti I, Minetti G, Castellan L, Rocco RMP, Pelosi P. Effects of positive end-expiratory pressure on lung recruitment, respiratory mechanics and intracranial pressure in mechanically ventilated brain injured patients. Front Physiol. 2021;12:711273.
Robba C, Ball L, Battaglini D, Iannuzzi F, Brunetti I, Fiaschi P, Zona G, Taccone FS, Messina A, Mongodi S, Pelosi P. Effects of positive end-expiratory pressure on lung ultrasound patterns and their correlation with intracranial pressure in mechanically ventilated brain injured patients. Crit Care. 2022;26:31. https://doi.org/10.1186/s13054-022-03903-7.
Robba C, Ball L, Battaglini D, Cardim D, Moncalvo E, Brunetti I, Bassetti M, Giacobbe DR, Vena A, Patroniti N, Rocco PRM, Matta BF, Pelosi P. Early effects of ventilatory rescue therapies on systemic and cerebral oxygenation in mechanically ventilated COVID-19 patients with acute respiratory distress syndrome: a prospective observational study. Crit Care. 2021;25:111. https://doi.org/10.1186/s13054-021-03537-1.
Dickson RP, Schultz MJ, van der Poll T, Schouten LR, Falkowski NR, Luth JE, Sjoding MW, Brown CA, Chanderraj R, Huffnagle GB, Bos LDJ, de Beer FM, Bos LD, Claushuis TA, Glas GJ, Horn J, Hoogendijk AJ, van Hooijdonk RT, Huson MA, de Jong MD, Juffermans NP, Lagrand WA, van der Poll T, Scicluna B, Schouten LR, Schultz MJ, van der Sluijs KF, Straat M, van Vught LA, Wieske L, Wiewel MA, Witteveen E. Lung microbiota predict clinical outcomes in critically Ill patients. Am J Respir Crit Care Med. 2020;201:555–63. https://doi.org/10.1164/rccm.201907-1487OC.
Kitsios GD, Yang H, Yang L, Qin S, Fitch A, Wang X-H, Fair K, Evankovich J, Bain W, Shah F, Li K, Methé B, Benos PV, Morris A, McVerry BJ. Respiratory tract dysbiosis is associated with worse outcomes in mechanically ventilated patients. Am J Respir Crit Care Med. 2020;202:1666–77. https://doi.org/10.1164/rccm.201912-2441OC.
Battaglini D, Siwicka Gieroba D, Brunetti I, Patroniti N, Bonatti G, Rocco PRM, Pelosi P, Robba C. Mechanical ventilation in neurocritical care setting: a clinical approach. Best Pract Res Clin Anaesthesiol. 2021;35:207–20. https://doi.org/10.1016/j.bpa.2020.09.001.
Zhang Y, Wang Z, Peng J, Gerner ST, Yin S, Jiang Y. Gut microbiota-brain interaction: an emerging immunotherapy for traumatic brain injury. Exp Neurol. 2021;337:113585. https://doi.org/10.1016/j.expneurol.2020.113585.
Battaglini D, Robba C, Lopes da Silva A, dos Santos SC, Leme Silva P, Dal Pizzol F, Pelosi P, Rocco PRM. Brain–heart interaction after acute ischemic stroke. Crit Care. 2020;24:163. https://doi.org/10.1186/s13054-020-02885-8.
Kerro A, Woods T, Chang JJ. Neurogenic stunned myocardium in subarachnoid hemorrhage. J Crit Care. 2017;38:27–34. https://doi.org/10.1016/j.jcrc.2016.10.010.
Buchholz S, Ward MR, Bhindi R, Nelson GIC, Figtree GA, Ward MR, Grieve SM, Bhindi R, Figtree GA, Grieve SM. Cardiac thrombi in stress (Tako-Tsubo) cardiomyopathy: more than an apical issue? Mayo Clin Proc. 2010;85:863–4. https://doi.org/10.4065/mcp.2010.0231.
Mazzeo AT, Micalizzi A, Mascia L, Scicolone A, Siracusano L. Brain–heart crosstalk: the many faces of stress-related cardiomyopathy syndromes in anaesthesia and intensive care. Br J Anaesth. 2014;112:803–15. https://doi.org/10.1093/bja/aeu046.
Zaroff JG, Pawlikowska L, Miss JC, Yarlagadda S, Ha C, Achrol A, Kwok P-Y, McCulloch CE, Lawton MT, Ko N, Smith W, Young WL. Adrenoceptor polymorphisms and the risk of cardiac injury and dysfunction after subarachnoid hemorrhage. Stroke. 2006;37:1680–5. https://doi.org/10.1161/01.STR.0000226461.52423.dd.
Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O’Gara P, Stuckey DJ, Nikolaev VO, Diakonov I, Pannell L, Gong H, Sun H, Peters NS, Petrou M, Zheng Z, Gorelik J, Lyon AR, Harding SE. High levels of circulating epinephrine trigger apical cardiodepression in a β 2 -adrenergic receptor/G i –dependent manner. Circulation. 2012;126:697–706. https://doi.org/10.1161/CIRCULATIONAHA.112.111591.
Griesdale DEG, Örtenwall V, Norena M, Wong H, Sekhon MS, Kolmodin L, Henderson WR, Dodek P. Adherence to guidelines for management of cerebral perfusion pressure and outcome in patients who have severe traumatic brain injury. J Crit Care. 2015;30:111–5. https://doi.org/10.1016/j.jcrc.2014.07.026.
Zhang L, Zhang B, Qi S. Impact of echocardiographic wall motion abnormality and cardiac biomarker elevation on outcome after subarachnoid hemorrhage: a meta-analysis. Neurosurg Rev. 2020;43:59–68. https://doi.org/10.1007/s10143-018-0985-6.
El-Menyar A, Goyal A, Latifi R, Al-Thani H, Frishman W. Brain-heart interactions in traumatic brain injury. Cardiol Rev. 2017;25:279–88. https://doi.org/10.1097/CRD.0000000000000167.
Dimitri GM, Beqiri E, Placek MM, Czosnyka M, Stocchetti N, Ercole A, Smielewski P, Lió P, Collaborators CENTER-TBI. Modeling brain-heart crosstalk information in patients with traumatic brain injury. Neurocrit Care. 2022;36:738–50. https://doi.org/10.1007/s12028-021-01353-7.
Funding
The authors have not received any financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
DAG, DB, and SDR contributed to the article conception and design; analysis and interpretation of literature; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement on accuracy and integrity of any part of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval/informed consent
We confirm that, for this work ethical guidelines, ethical approvals (institutional review board) and the use of informed consent were not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
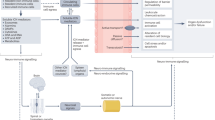
The original article has been corrected. In this article the Figures 2 and 3 were wrongly numbered and not the right figure for those legends. Fig.2 should have been Fig.3 and vice versa.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Battaglini, D., De Rosa, S. & Godoy, D.A. Crosstalk Between the Nervous System and Systemic Organs in Acute Brain Injury. Neurocrit Care 40, 337–348 (2024). https://doi.org/10.1007/s12028-023-01725-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01725-1