Abstract
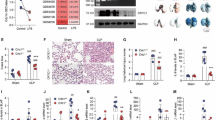
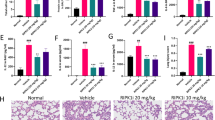
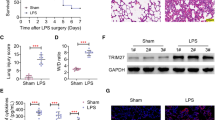
Acute respiratory distress syndrome (ARDS)/acute lung injury (ALI) is a severe complication of sepsis characterized by acute respiratory distress, hypoxemia, and diffuse bilateral pulmonary infiltrates. The regulation of RIPK1 is an important part of the inflammatory response, and cIAP1/2 serves as the E3 ubiquitin ligase for RIPK1. In this study, we investigated the effect and mechanism of cIAP1/2 inhibition on sepsis-induced lung injury. Our results showed that cIAP1/2 inhibition can alleviate sepsis-induced lung injury and reduce the inflammatory response, which is accompanied by downregulation of RIPK1 phosphorylation and ubiquitination. Additionally, cIAP1/2 inhibition led to the up-regulation of programmed cell death, including apoptosis, necroptosis, and pyroptosis, and inhibiting these three cell death pathways can further reduce the inflammatory response, which is similar to the recently discovered programmed cell death pathway PANoptosis. Our findings suggest that cIAP1/2 and PANoptosis inhibition may be a new strategy for treating sepsis-induced lung injury and provide important references for further exploring the mechanism of sepsis-induced lung injury and identifying new therapeutic targets.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Dushianthan A, Grocott MPW, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J. 2011;87:612–22.
Botros L, Pronk MCA, Juschten J, Liddle J, Morsing SKH, van Buul JD, et al. Bosutinib prevents vascular leakage by reducing focal adhesion turnover and reinforcing junctional integrity. J Cell Sci. 2020;133:jcs240077.
Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science [Internet]. 1998 ;281:1305–8. https://www.science.org/doi/https://doi.org/10.1126/science.281.5381.1305. [cited 2023 Jun 9].
Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP, Tumor necrosis factor, receptor and Fas signaling mechanismS. Annual Review of Immunology [Internet]. 1999;17:331–67. https://doi.org/10.1146/annurev.immunol.17.1.331. [cited 2023 Jun 9].
Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:11778–83.
Wong WW-L, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A et al. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood [Internet]. 2014;123:2562–72. https://www.sciencedirect.com/science/article/pii/S0006497120357529. [cited 2022 Sep 29].
Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–74.
Coskun M, Olsen J, Seidelin JB, Nielsen OH. MAP kinases in inflammatory bowel disease. Clin Chim Acta. 2011;412:513–20.
Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg Axis in Autoimmune disorders. Molecules. 2017;22:134.
Kawalkowska JZ, Ogbechi J, Venables PJ, Williams RO. cIAP1/2 inhibition synergizes with TNF inhibition in autoimmunity by down-regulating IL-17A and inducing Tregs. Sci Adv. 2019;5:eaaw5422.
Liu Z, Mar KB, Hanners NW, Perelman SS, Kanchwala M, Xing C, et al. A NIK-SIX signalling axis controls inflammation by targeted silencing of non-canonical NF-κB. Nature. 2019;568:249–53.
Jensen S, Seidelin JB, LaCasse EC, Nielsen OH. SMAC mimetics and RIPK inhibitors as therapeutics for chronic inflammatory diseases. Sci Signal. 2020;13:eaax8295.
Seidelin JB, Vainer B, Andresen L, Nielsen OH. Upregulation of cIAP2 in regenerating colonocytes in ulcerative colitis. Virchows Arch. 2007;451:1031–8.
Seidelin JB, Larsen S, Linnemann D, Vainer B, Coskun M, Troelsen JT, et al. Cellular inhibitor of apoptosis protein 2 controls human colonic epithelial restitution, migration, and Rac1 activation. Am J Physiol Gastrointest Liver Physiol. 2015;308:G92–99.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9.
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S et al. Fas triggers an alternative, caspase-8–independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol [Internet]. 2000;1:489–95. http://www.nature.com/articles/ni1200_489. [cited 2022 Dec 1].
Newton K, Multitasking kinase RIPK1 Regulates cell death and inflammation. Cold Spring Harb Perspect Biol [Internet]. 2020;12:a036368. http://cshperspectives.cshlp.org/content/12/3/a036368. [cited 2023 Feb 2].
Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17:3261–70.
Hennessy EJ, Adam A, Aquila BM, Castriotta LM, Cook D, Hattersley M et al. Discovery of a novel class of dimeric smac mimetics as potent IAP antagonists resulting in a clinical candidate for the treatment of cancer (AZD5582). J Med Chem [Internet]. 2013;56:9897–919. https://doi.org/10.1021/jm401075x. [cited 2022 Apr 10].
Wang Q, Ye Q, Xi X, Cao X, Wang X, Zhang M et al. KW2449 ameliorates collagen-induced arthritis by inhibiting RIPK1-dependent necroptosis. Front Immunol [Internet]. 2023;14:1135014. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10040599/. [cited 2023 Aug 22].
Liu Y, Xu Q, Wang Y, Liang T, Li X, Wang D, et al. Necroptosis is active and contributes to intestinal injury in a piglet model with lipopolysaccharide challenge. Cell Death Dis. 2021;12:62.
Wang L, Wang T, Li H, Liu Q, Zhang Z, Xie W, et al. Receptor interacting protein 3-Mediated necroptosis promotes Lipopolysaccharide-Induced inflammation and Acute Respiratory Distress Syndrome in mice. PLoS ONE. 2016;11:e0155723.
Liu X, Chen J, Li Z, Gao N, Zhang G. CIAP1/2 can regulate the inflammatory response and lung injury induced by apoptosis in septic rats. J Invest Med [Internet]. 2023;10815589231207102. https://doi.org/10.1177/10815589231207102. [cited 2023 Nov 29].
Abraham MN, Jimenez DM, Fernandes TD, Deutschman CS. Cecal Ligation and puncture alters glucocorticoid receptor expression. Crit Care Med. 2018;46:e797–804.
Chen D-Q, Wang Y-N, Vaziri ND, Chen L, Hu H-H, Zhao Y-Y. Poricoic acid a activates AMPK to attenuate fibroblast activation and abnormal extracellular matrix remodelling in renal fibrosis. Phytomedicine. 2020;72:153232.
Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–38.
Lee EH, Shin MH, Gi M, Park J, Song D, Hyun Y-M, et al. Inhibition of Pendrin by a small molecule reduces Lipopolysaccharide-induced acute lung Injury. Theranostics. 2020;10:9913–22.
Amin P, Florez M, Najafov A, Pan H, Geng J, Ofengeim D et al. Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFα-mediated apoptosis. Proc Natl Acad Sci USA [Internet]. 2018;115. https://doi.org/10.1073/pnas.1806973115. [cited 2022 May 9].
Lorente JA, Marshall JC. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock (Augusta Ga). 2005;24(Suppl 1):107–19.
Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Resp Crit Care. 2002;166:1197–205.
Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Sci (N Y NY). 1985;229:869–71.
Natanson C, Esposito CJ, Banks SM. The sirens’ songs of confirmatory sepsis trials: selection bias and sampling error. Crit Care Med. 1998;26:1927–31.
Qiu P, Cui X, Barochia A, Li Y, Natanson C, Eichacker PQ. The evolving experience with therapeutic TNF inhibition in sepsis: considering the potential influence of risk of death. Expert Opin Invest Drugs [Internet]. 2011;20:1555–64. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3523300/. [cited 2024 Apr 13].
Wang Q, Fan D, Xia Y, Ye Q, Xi X, Zhang G et al. The latest information on the RIPK1 post-translational modifications and functions. Biomedicine & Pharmacotherapy [Internet]. 2021;142:112082. https://www.sciencedirect.com/science/article/pii/S0753332221008659. [cited 2021 Sep 5].
Delanghe T, Dondelinger Y, Bertrand MJM. RIPK1 kinase-dependent death: a Symphony of Phosphorylation events. Trends Cell Biol. 2020;30:189–200.
Simpson DS, Gabrielyan A, Feltham R. RIPK1 ubiquitination: evidence, correlations and the undefined. Seminars in cell & developmental biology [Internet]. 2021;109:76–85. https://linkinghub.elsevier.com/retrieve/pii/S1084952119302411. [cited 2022 Aug 23].
Wang Y, Kanneganti T-D. From pyroptosis, apoptosis and necroptosis to PANoptosis: a mechanistic compendium of programmed cell death pathways. Comput Struct Biotechnol J [Internet]. 2021;19:4641–57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8405902/. [cited 2023 Feb 19].
Flexible usage and interconnectivity of diverse cell. death pathways protect against intracellular infection. Immunity [Internet]. 2020;53:533–547.e7. https://www.sciencedirect.com/science/article/pii/S1074761320302843. [cited 2024 Jan 19].
Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B, et al. Identification of the PANoptosome: a molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol. 2020;10:237.
Acknowledgements
We would like to express our gratitude to the Beijing Key Lab for Immune-Mediated Inflammatory Diseases of China-Japan Friendship Hospital for providing the cell culture environment and to the Institute of Clinical Medical Research of China-Japan Friendship Hospital for their laboratory support.
Funding
This work was supported by the National Natural Science Foundation of China [no. 82272196] and National Key R&D Program of China [no. 2023YFC3011804].
Author information
Authors and Affiliations
Contributions
Xiaoyu Liu and Yan Li are co-first authors, designed this study, drafted the manuscript, and contributed equally to this study. Jie Chen performed the animal model and sections. Xiaoyu Liu performed cell culture and flow cytometry. Weijian Zhang conducted the data analysis. Nan Gao performed the WB and qPCR. Guoqiang Zhang and Cheng Xiao revised this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This work is an original creation and not a salami publication of previously completed work. All sources used in this work have been properly cited and acknowledged.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Affiliations 2, 3 and 4 was contained mistakes and has been corrected.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Li, Y., Zhang, W. et al. Inhibition of cIAP1/2 reduces RIPK1 phosphorylation in pulmonary endothelial cells and alleviate sepsis-induced lung injury and inflammatory response. Immunol Res (2024). https://doi.org/10.1007/s12026-024-09491-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12026-024-09491-8