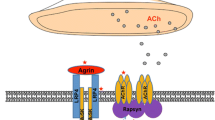
Abstract
Myasthenia gravis (MG) is a type of muscle paralysis created by immune responses against acetylcholine receptor proteins in neuromuscular synapses. This disease is characterized by muscle weakness, especially ocular weakness symptoms that could be ptosis (fall of the upper eyelid) or diplopia (double vision of a single object). Some patients also identified with speech and swallowing problems. The main goals of MG therapeutic approaches are to achieve remission, reduce symptoms, and improve life quality. Recently, other studies have revealed the potential role of various microRNAs (miRNAs) in the development of MG through different mechanisms and have proposed these molecules as effective biomarkers for the treatment of MG. This review was aimed at providing an overview of the critical regulatory roles of various miRNAs in the pathogenesis of this autoimmune disease focusing on human MG studies and the interaction between different miRNAs with important cytokines and immune cells during the development of this autoimmune disease.




Similar content being viewed by others
Data availability
The authors can confirm that all relevant data are included in the article. Dataset(s) are derived from public resources and made available with the article (references).
Code availability
Not applicable.
References
McCorry LK. Physiology of the autonomic nervous system. Am J Pharm Educ. 2007;71:78. https://doi.org/10.5688/aj710478.
Dresser L, Wlodarski R, Rezania K, Soliven B. Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med. 2021;10(11):2235. https://doi.org/10.3390/jcm10112235.
Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. Myasthenia gravis - autoantibody characteristics and their implications for therapy. Nat Rev Neurol Engl. 2016;12:259–68. https://doi.org/10.1038/nrneurol.2016.44.
Thanvi BR, Lo TCN. Update on myasthenia gravis. Postgrad Med J. 2004;80:690–700. https://doi.org/10.1136/pgmj.2004.018903.
Punga AR, Maddison P, Heckmann JM, Guptill JT, Evoli A. Epidemiology, diagnostics, and biomarkers of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022;21:176–88. https://doi.org/10.1016/S1474-4422(21)00297-0.
Berrih-Aknin S, Frenkian-Cuvelier M, Eymard B. Diagnostic and clinical classification of autoimmune myasthenia gravis. J Autoimmun Engl. 2014;48–49:143–8. https://doi.org/10.1016/j.jaut.2014.01.003.
Renjen PN. Subgroup Classification of Myasthenia Gravis. Ann Indian Acad Neurol. 2021;24:300. https://doi.org/10.4103/aian.AIAN_237_20.
Szczudlik P, Szyluk B, Lipowska M, Ryniewicz B, Kubiszewska J, Dutkiewicz M, et al. Antititin antibody in early- and late-onset myasthenia gravis. Acta neurologica Scandinavica Denmark. 2014;130:229–33. https://doi.org/10.1111/ane.12271.
Kufukihara K, Watanabe Y, Inagaki T, Takamatsu K, Nakane S, Nakahara J, et al. Cytometric cell-based assays for anti-striational antibodies in myasthenia gravis with myositis and/or myocarditis. Sci Rep. 2019;9:5284. https://doi.org/10.1038/s41598-019-41730-z.
Narayanaswami P, Sanders DB, Wolfe G, Benatar M, Cea G, Evoli A, et al. International consensus guidance for management of myasthenia gravis: 2020 Update. Neurology. 2021;96:114–22. https://doi.org/10.1212/WNL.0000000000011124.
Gilhus NE. Myasthenia Gravis. The New England journal of medicine. United States. 2016;375:2570–81. https://doi.org/10.1056/NEJMra1602678.
Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–42. https://doi.org/10.1016/j.cell.2008.10.002.
Bubuioc A-M, Kudebayeva A, Turuspekova S, Lisnic V, Leone MA. The epidemiology of myasthenia gravis. J med life. 2021;14(1):7–16.
Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. 2020;11:212. https://doi.org/10.3389/fimmu.2020.00212.
Gilhus NE, Owe JF, Hoff JM, Romi F, Skeie GO, Aarli JA. Myasthenia gravis: a review of available treatment approaches. Autoimmune Dis. 2011;2011:847393. https://doi.org/10.4061/2011/847393.
Nair AG, Patil-Chhablani P, Venkatramani DV, Gandhi RA. Ocular myasthenia gravis: a review. Indian J Ophthalmol. 2014;62:985–91. https://doi.org/10.4103/0301-4738.145987.
Jordan A, Freimer M. Recent advances in understanding and managing myasthenia gravis. F1000Research. 2018;7. https://doi.org/10.12688/f1000research.15973.1
Melzer N, Ruck T, Fuhr P, Gold R, Hohlfeld R, Marx A, et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J Neurol. 2016;263:1473–94. https://doi.org/10.1007/s00415-016-8045-z.
Evoli A, Antonini G, Antozzi C, DiMuzio A, Habetswallner F, Iani C, et al. Italian recommendations for the diagnosis and treatment of myasthenia gravis. Neurol Sci. 2019;40:1111–24. https://doi.org/10.1007/s10072-019-03746-1.
Peragallo JH, Bitrian E, Kupersmith MJ, Zimprich F, Whittaker TJ, Lee MS, et al. Relationship between age, gender, and race in patients presenting with myasthenia gravis with only ocular manifestations. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc United States. 2016;36:29–32. https://doi.org/10.1097/WNO.0000000000000276.
Schneider-Gold C, Hagenacker T, Melzer N, Ruck T. Understanding the burden of refractory myasthenia gravis. Ther Adv Neurol Disord. 2019;12:1756286419832242. https://doi.org/10.1177/1756286419832242.
Alkhawajah NM, Oger J. Treatment of myasthenia gravis in the aged. Drugs aging New Zealand. 2015;32:689–97. https://doi.org/10.1007/s40266-015-0297-2.
Pfeiffer HCV, Løkkegaard A, Zoetmulder M, Friberg L, Werdelin L. Cognitive impairment in early-stage non-demented Parkinson’s disease patients. Acta neurologica Scandinavica Denmark. 2014;129:307–18. https://doi.org/10.1111/ane.12189.
Vitturi BK, Kim AIH, Mitre LP, Pellegrinelli A, Valerio BCO. Social, professional and neuropsychiatric outcomes in patients with myasthenia gravis. Neurol Sci. 2021;42:167–73. https://doi.org/10.1007/s10072-020-04528-w.
Meriggioli MN, Sanders DB. Muscle autoantibodies in myasthenia gravis: beyond diagnosis? Expert Rev Clin Immunol. 2012;8:427–38. https://doi.org/10.1586/eci.12.34.
Truffault F, de Montpreville V, Eymard B, Sharshar T, Le Panse R, Berrih-Aknin S. Thymic germinal centers and corticosteroids in myasthenia gravis: an immunopathological study in 1035 cases and a critical review. Clinical reviews in allergy & immunology. United States. 2017;52:108–24. https://doi.org/10.1007/s12016-016-8558-3.
Vrolix K, Fraussen J, Losen M, Stevens J, Lazaridis K, Molenaar PC, et al. Clonal heterogeneity of thymic B cells from early-onset myasthenia gravis patients with antibodies against the acetylcholine receptor. J Autoimmun Engl. 2014;52:101–12. https://doi.org/10.1016/j.jaut.2013.12.008.
Balasa B, Sarvetnick N. Is pathogenic humoral autoimmunity a Th1 response? Lessons from (for) myasthenia gravis. Immunol today Engl. 2000;21:19–23. https://doi.org/10.1016/S0167-5699(99)01553-4.
Vinuesa CG, Linterman MA, Yu D, MacLennan ICM. Follicular helper T cells. Annual Rev Immun United States. 2016;34:335–68. https://doi.org/10.1146/annurev-immunol-041015-055605.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology United States. 2009;27:485–517. https://doi.org/10.1146/annurev.immunol.021908.132710.
Masuda M, Matsumoto M, Tanaka S, Nakajima K, Yamada N, Ido N, et al. Clinical implication of peripheral CD4+CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. J Neuroimmunol Netherlands. 2010;225:123–31. https://doi.org/10.1016/j.jneuroim.2010.03.016.
Çebi M, Durmus H, Aysal F, Özkan B, Gül GE, Çakar A, et al. CD4(+) T cells of myasthenia gravis patients are characterized by increased IL-21, IL-4, and IL-17A productions and higher presence of PD-1 and ICOS. Front Immunol. 2020;11:809. https://doi.org/10.3389/fimmu.2020.00809.
Yousefi Z, Mirsanei Z, Bitaraf FS, Mahdavi S, Mirzaii M, Jafari R. Dose-dependent effects of oleuropein administration on regulatory T-cells in patients with rheumatoid arthritis: an in vitro approach. Int J Immunopathol Pharmacol. 2022;36:3946320221086084. https://doi.org/10.1177/03946320221086084.
Sabre L, Punga T, Punga AR. Circulating miRNAs as potential biomarkers in myasthenia gravis: tools for personalized medicine. Front Immunol. 2020;11:213. https://doi.org/10.3389/fimmu.2020.00213.
Golabi M, Fathi F, Samadi M, Hesamian MS, Eskandari N. Identification of potential biomarkers in the peripheral blood mononuclear cells of relapsing-remitting multiple sclerosis patients. Inflamm United States. 2022;45:1815–28. https://doi.org/10.3389/fimmu.2020.00213.
Kabekkodu SP, Shukla V, Varghese VK, D’Souza J, Chakrabarty S, Satyamoorthy K. Clustered miRNAs and their role in biological functions and diseases. Biological reviews of the Cambridge Philosophical Society. Biol Rev. 2018;93:1955–86. https://doi.org/10.1111/brv.12428.
Asadi M, Shanehbandi D, Zafari V, Khaze V, Somi MH, Hashemzadeh S. Transcript level of microRNA processing elements in gastric cancer. J Gastrointest Cancer United States. 2019;50:855–9. https://doi.org/10.1007/s12029-018-0154-8.
Long H, Wang X, Chen Y, Wang L, Zhao M, Lu Q. Dysregulation of microRNAs in autoimmune diseases: pathogenesis, biomarkers and potential therapeutic targets. Cancer letters Ireland. 2018;428:90–103. https://doi.org/10.1016/j.canlet.2018.04.016.
Cron MA, Guillochon É, Kusner L, Le Panse R. Role of miRNAs in normal and myasthenia gravis thymus. Front Immunol. 2020;11:1074. https://doi.org/10.3389/fimmu.2020.01074.
Montazeri M, Eskandari N, Mansouri R. Evaluation of the expressed miR-129 and miR-549a in patients with multiple sclerosis. Adv Biomed Res. 2021;10:48. https://doi.org/10.4103/abr.abr_268_20.
Wang L, Zhang L. Emerging roles of dysregulated microRNAs in myasthenia gravis. Front Neurosci. 2020;14:507. https://doi.org/10.3389/fnins.2020.00507.
Punga AR, Andersson M, Alimohammadi M, Punga T. Disease specific signature of circulating miR-150-5p and miR-21-5p in myasthenia gravis patients. J Neurol Sci Netherlands. 2015;356:90–6. https://doi.org/10.1016/j.jns.2015.06.019.
Punga T, Le Panse R, Andersson M, Truffault F, Berrih-Aknin S, Punga AR. Circulating miRNAs in myasthenia gravis: miR-150-5p as a new potential biomarker. Ann Clin Trans Neurol. 2014;1:49–58. https://doi.org/10.1002/acn3.24.
Punga AR, Punga T. Circulating microRNAs as potential biomarkers in myasthenia gravis patients. Ann New York Acad Sci United States. 2018;1412:33–40. https://doi.org/10.1111/nyas.13510.
Sabre L, Maddison P, Sadalage G, Ambrose PA, Punga AR. Circulating microRNA miR-21-5p, miR-150-5p and miR-30e-5p correlate with clinical status in late onset myasthenia gravis. J Neuroimmunol Netherlands. 2018;321:164–70. https://doi.org/10.1016/j.jneuroim.2018.05.003.
Wang J, Guan X, Guo F, Zhou J, Chang A, Sun B, et al. miR-30e reciprocally regulates the differentiation of adipocytes and osteoblasts by directly targeting low-density lipoprotein receptor-related protein 6. Cell Death Dis. 2013;4:e845. https://doi.org/10.1038/cddis.2013.356.
Sabre L, Guptill JT, Russo M, Juel VC, Massey JM, Howard JFJ, et al. Circulating microRNA plasma profile in MuSK+ myasthenia gravis. J Neuroimmunol. 2018;325:87–91. https://doi.org/10.1016/j.jneuroim.2018.10.003.
Punga T, Bartoccioni E, Lewandowska M, Damato V, Evoli A, Punga AR. Disease specific enrichment of circulating let-7 family microRNA in MuSK+ myasthenia gravis. J Neuroimmunol Netherlands. 2016;292:21–6. https://doi.org/10.1016/j.jneuroim.2016.01.003.
Pianta S, Bonassi Signoroni P, Muradore I, Rodrigues MF, Rossi D, Silini A, et al. Amniotic membrane mesenchymal cells-derived factors skew T cell polarization toward Treg and downregulate Th1 and Th17 cells subsets. Stem Cell Rev Rep. 2015;11:394–407. https://doi.org/10.1007/s12015-014-9558-4.
Liu X, Luo M, Meng H, Zeng Q, Xu L, Hu B, et al. MiR-181a regulates CD4(+) T cell activation and differentiation by targeting IL-2 in the pathogenesis of myasthenia gravis. Germany: European journal of immunology; 2019. https://doi.org/10.1002/eji.201848007.
Bortone F, Scandiffio L, Marcuzzo S, Bonanno S, Frangiamore R, Motta T, et al. miR-146a in myasthenia gravis thymus bridges innate immunity with autoimmunity and is linked to therapeutic effects of corticosteroids. Frontiers in Immunology. 2020;11. https://doi.org/10.3389/fimmu.2020.00142
Wang Y-Z, Tian F-F, Yan M, Zhang J-M, Liu Q, Lu J-Y, et al. Delivery of an miR155 inhibitor by anti-CD20 single-chain antibody into B cells reduces the acetylcholine receptor-specific autoantibodies and ameliorates experimental autoimmune myasthenia gravis. Clin Exp Immunol. 2014;176:207–21. https://doi.org/10.1111/cei.12265.
Yeh J-H, Wang S-H, Chien P-J, Shih C-M, Chiu H-C. Changes in serum cytokine levels during plasmapheresis in patients with myasthenia gravis. Eur J Neurol Engl. 2009;16:1318–22. https://doi.org/10.1111/j.1468-1331.2009.02729.x.
Matusevicius D, Navikas V, Palasik W, Pirskanen R, Fredrikson S, Link H. Tumor necrosis factor-alpha, lymphotoxin, interleukin (IL)-6, IL-10, IL-12 and perforin mRNA expression in mononuclear cells in response to acetylcholine receptor is augmented in myasthenia gravis. J Neuroimmunol Netherlands. 1996;71:191–8. https://doi.org/10.1016/S0165-5728(96)00152-X.
Jiang L, Cheng Z, Qiu S, Que Z, Bao W, Jiang C, et al. Altered let-7 expression in Myasthenia gravis and let-7c mediated regulation of IL-10 by directly targeting IL-10 in Jurkat cells. Int immunopharmacol Netherlands. 2012;14:217–23. https://doi.org/10.1016/j.intimp.2012.07.003.
Cheng Z, Qiu S, Jiang L, Zhang A, Bao W, Liu P, et al. MiR-320a is downregulated in patients with myasthenia gravis and modulates inflammatory cytokines production by targeting mitogen-activated protein kinase 1. J Clin Immunol Netherlands. 2013;33:567–76. https://doi.org/10.1007/s10875-012-9834-5.
Shi L, Liu T, Zhang M, Guo Y, Song C, Song D, et al (2015) miR-15b is Downregulated in Myasthenia Gravis Patients and Directly Regulates the Expression of Interleukin-15 (IL-15) in Experimental Myasthenia Gravis Mice. Medical science monitor : international medical journal of experimental and clinical research. 21:1774–80. https://doi.org/10.12659/MSM.893458
Roche JC, Capablo JL, Larrad L, Gervas-Arruga J, Ara JR, Sánchez A, et al. Increased serum interleukin-17 levels in patients with myasthenia gravis. Muscle Nerve United States. 2011;44:278–80. https://doi.org/10.1002/mus.22070.
Zhang Y, Guo M, Xin N, Shao Z, Zhang X, Zhang Y, et al. Decreased microRNA miR-181c expression in peripheral blood mononuclear cells correlates with elevated serum levels of IL-7 and IL-17 in patients with myasthenia gravis. Clin Exp Med Italy. 2016;16:413–21. https://doi.org/10.1007/s10238-015-0358-1.
Chunjie N, Huijuan N, Zhao Y, Jianzhao W, Xiaojian Z. Disease-specific signature of serum miR-20b and its targets IL-8 and IL-25, in myasthenia gravis patients. Eur Cytokine Network France. 2015;26:61–6. https://doi.org/10.1684/ecn.2015.0367.
Wang J, Zheng S, Xin N, Dou C, Fu L, Zhang X, et al. Identification of novel MicroRNA signatures linked to experimental autoimmune myasthenia gravis pathogenesis: down-regulated miR-145 promotes pathogenetic Th17 cell response. J Neuroimmune Pharmacol United States. 2013;8:1287–302. https://doi.org/10.1007/s11481-013-9498-9.
Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol (Baltimore, Md : 1950) United States. 2002;168:3195–204. https://doi.org/10.4049/jimmunol.168.7.3195.
Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine & Growth Factor Rev England. 2015;26:311–27. https://doi.org/10.1016/j.cytogfr.2014.11.009.
Liu X-F, Wang R-Q, Hu B, Luo M-C, Zeng Q-M, Zhou H, et al (2016) MiR-15a contributes abnormal immune response in myasthenia gravis by targeting CXCL10 Clinical immunology (Orlando, Fla) United States 164:106–13 https://doi.org/10.1016/j.clim.2015.12.009
Li J, Qiu D, Chen Z, Du W, Liu J, Mo X. miR-548k regulates CXCL13 expression in myasthenia gravis patients with thymic hyperplasia and in Jurkat cells. J Neuroimmunol Netherlands. 2018;320:125–32. https://doi.org/10.1016/j.jneuroim.2018.03.021.
Li J, Qiu D, Chen Z, Du W, Liu J, Mo X. Altered expression of miR-125a-5p in thymoma-associated myasthenia gravis and its down-regulation of foxp3 expression in Jurkat cells. Immunol lett Netherlands. 2016;172:47–55. https://doi.org/10.1016/j.imlet.2016.02.005.
Ao W, Tian C, He X, Hu Y, Wang W, Liu Y. Upregulation of miR150–5p in generalized myasthenia gravis patients is associated with decreased serum levels of IL-17 and increased serum levels of IL-10. Biomed papers Med Fac of the University Palacky, Olomouc, Czechoslovakia Czech Republic. 2020;164:57–62. https://doi.org/10.5507/bp.2019.009.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MG, ZY, MJ, MM, and NE conceptualized the study and wrote the manuscript. SB and BG contributed to edit an drafting of the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Golabi, M., Yousefi, Z., Jafarinia, M. et al. miRNAs as the important regulators of myasthenia gravis: involvement of major cytokines and immune cells. Immunol Res 71, 153–163 (2023). https://doi.org/10.1007/s12026-022-09342-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09342-4