Abstract
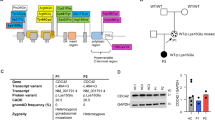
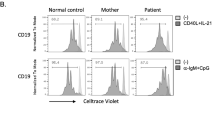
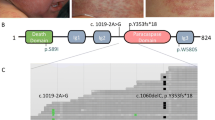
Severe combined immunodeficiency (SCID) disorders compromise lymphocyte numbers and/or function. One subset of SCID typically affects T cell and Natural Killer (NK) cell development in tandem (T−B+NK−) due to mutations arising in the genes encoding the common γ chain or Janus Kinase 3 (JAK3). In rare circumstances, mutations in the JAK3 gene have been reported to cause atypical SCID that selectively affects T cells (T−B+NK+). Here we describe a case involving a female infant who was referred to our institution on day nine of life following an abnormal newborn screen result for T−SCID. Immunological assessments revealed a T−B+NK+ phenotype and molecular analyses, including whole exome sequencing, identified compound heterozygous JAK3 variants (R117C and E658K). Pre-transplant phosflow analyses revealed a persistent IL-7 signaling defect, based on phospho-STAT5 measurements, only in CD8 but not CD4 T cells. Intriguingly, phospho-STAT5 signals in response to IL-2 stimulation were not affected in either CD4 or CD8 T cells. The pre-transplant clinical course was unremarkable, and the patient received a cord-blood stem cell transplant on day 716 of life. Post-transplant monitoring revealed that despite normalization of lymphocyte counts, the CD8 T cell-restricted IL-7 signaling defect was still evident at day 627 post-transplant (phospho-STAT5 signal in CD8 T cells was > 60% reduced compared with CD4 T cells). The post-transplant clinical course has also been complicated by identification of autoimmune responses and likely GVHD-induced ichthyosis. To the best of our knowledge, this report represents the third case of JAK3-associated atypical SCID reported in the literature.







Similar content being viewed by others
References
Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312(7):729–38. https://doi.org/10.1001/jama.2014.9132.
Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133(4):1092–8. https://doi.org/10.1016/j.jaci.2013.09.044.
Mamcarz E, Zhou S, Lockey T, Abdelsamed H, Cross SJ, Kang G, et al. Lentiviral gene therapy combined with low-dose busulfan in infants with SCID-X1. N Engl J Med. 2019;380(16):1525–34. https://doi.org/10.1056/NEJMoa1815408.
Immune Deficiency Foundation (IDF) SCID Newborn screening campaign. Immune Deficiency Foundation, Towson, MD. 2019. https://primaryimmune.org/idf-advocacy-center/idf-scid-newborn-screening-campaign. Accessed March 3, 2019 2019.
Bausch-Jurken MT, Verbsky JW, Routes JM. Newborn screening for severe combined immunodeficiency-a history of the TREC assay. Int J Neonatal Screening. 2017;3(14). https://doi.org/10.3390/ijns3020014.
Candotti F, Oakes SA, Johnston JA, Giliani S, Schumacher RF, Mella P, et al. Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood. 1997;90(10):3996–4003.
Brugnoni D, Notarangelo LD, Sottini A, Airo P, Pennacchio M, Mazzolari E, et al. Development of autologous, oligoclonal, poorly functioning T lymphocytes in a patient with autosomal recessive severe combined immunodeficiency caused by defects of the Jak3 tyrosine kinase. Blood. 1998;91(3):949–55.
Li J, Nara H, Rahman M, Juliana FM, Araki A, Asao H. Impaired IL-7 signaling may explain a case of atypical JAK3-SCID. Cytokine. 2010;49(2):221–8. https://doi.org/10.1016/j.cyto.2009.09.009.
Farnault L, Chambost H, Michel G, Thuret I, de Saint BG, Fischer A, et al. Persistence of natural killer cells with expansion of a hypofunctional CD56-CD16+KIR+NKG2C+ subset in a patient with atypical Janus kinase 3-deficient severe combined immunodeficiency. J Allergy Clin Immunol. 2013;131(4):1230–3, 3 e1–2. https://doi.org/10.1016/j.jaci.2012.08.047.
Khanolkar A, Wilks JD, Jennings LJ, Davies JL, Zollett JA, Lott LL, et al. Signaling impairments in maternal T cells engrafted in an infant with a novel IL-2 receptor gamma mutation. J Allergy Clin Immunol. 2015;135(4):1093–6 e8. https://doi.org/10.1016/j.jaci.2015.02.012.
Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. https://doi.org/10.1146/annurev.immunol.22.012703.104702.
Irish JM, Myklebust JH, Alizadeh AA, Houot R, Sharman JP, Czerwinski DK, et al. B-cell signaling networks reveal a negative prognostic human lymphoma cell subset that emerges during tumor progression. Proc Natl Acad Sci U S A. 2010;107(29):12747–54. https://doi.org/10.1073/pnas.1002057107.
Muul LM, Heine, G., Silvin, C., James, S.P., Candotti, F., Radbruch, A., Worm, M. Measurement of proliferative responses of cultured lymphocytes. Current Protocols in Immunology. John Wiley & Sons, Inc; 2011. p. 94:7.10.1–7.10.26.
Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30(12):e57.
McDonald-McGinn DM, Fahiminiya S, Revil T, Nowakowska BA, Suhl J, Bailey A, et al. Hemizygous mutations in SNAP29 unmask autosomal recessive conditions and contribute to atypical findings in patients with 22q11.2DS. J Med Genet. 2013;50(2):80–90. https://doi.org/10.1136/jmedgenet-2012-101320.
Onoe T, Kalscheuer H, Chittenden M, Zhao G, Yang YG, Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APCs. J Immunol. 2010;184(12):6756–65. https://doi.org/10.4049/jimmunol.0901711.
Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24(3):209–17. https://doi.org/10.1016/j.smim.2012.04.010.
Fink PJ. The biology of recent thymic emigrants. Annu Rev Immunol. 2013;31:31–50. https://doi.org/10.1146/annurev-immunol-032712-100010.
Robin NH, Shprintzen RJ. Defining the clinical spectrum of deletion 22q11.2. J Pediatr. 2005;147(1):90–6. https://doi.org/10.1016/j.jpeds.2005.03.007.
Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–8. https://doi.org/10.1038/ni1009.
Hou J, Schindler U, Henzel WJ, Wong SC, McKnight SL. Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995;2(4):321–9.
Notarangelo LD, Mella P, Jones A, de Saint BG, Savoldi G, Cranston T, et al. Mutations in severe combined immune deficiency (SCID) due to JAK3 deficiency. Hum Mutat. 2001;18(4):255–63. https://doi.org/10.1002/humu.1188.
Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206(1):25–34. https://doi.org/10.1084/jem.20082013.
Mishra J, Karanki SS, Kumar N. Identification of molecular switch regulating interactions of Janus kinase 3 with cytoskeletal proteins. J Biol Chem. 2012;287(49):41386–91. https://doi.org/10.1074/jbc.C112.363507.
Mishra J, Kumar N. Adapter protein Shc regulates Janus kinase 3 phosphorylation. J Biol Chem. 2014;289(23):15951–6. https://doi.org/10.1074/jbc.C113.527523.
Henriques CM, Rino J, Nibbs RJ, Graham GJ, Barata JT. IL-7 induces rapid clathrin-mediated internalization and JAK3-dependent degradation of IL-7Ralpha in T cells. Blood. 2010;115(16):3269–77. https://doi.org/10.1182/blood-2009-10-246876.
Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21(2):289–302. https://doi.org/10.1016/j.immuni.2004.07.016
Kim HR, Hwang KA, Kim KC, Kang I. Down-regulation of IL-7Ralpha expression in human T cells via DNA methylation. J Immunol. 2007;178(9):5473–9.
Kim HR, Hwang KA, Kang I. Dual roles of IL-15 in maintaining IL-7RalphalowCCR7- memory CD8+ T cells in humans via recovering the phosphatidylinositol 3-kinase/AKT pathway. J Immunol. 2007;179(10):6734–40. https://doi.org/10.4049/jimmunol.179.10.6734.
Acknowledgments
We would like to express our sincere gratitude to the following individuals for their help with the whole exome sequencing assay: Drs. Carrie L. Lucas, PhD, and Jean-Marie Carpier, PhD (Yale University Immunobiology program), and Drs. Weizhen Ji, PhD, and Saquib A. Lakhani, MD (Yale University Pediatric Genomic Discovery Program).
Funding
This work was partly supported by philanthropic funds provided by the Jeffrey Modell Foundation to the Ann and Robert H. Lurie Children’s Hospital of Chicago.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 210 kb)
Rights and permissions
About this article
Cite this article
Khanolkar, A., Wilks, J.D., Liu, G. et al. A case of aberrant CD8 T cell–restricted IL-7 signaling with a Janus kinase 3 defect–associated atypical severe combined immunodeficiency. Immunol Res 68, 13–27 (2020). https://doi.org/10.1007/s12026-020-09123-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-020-09123-x