Abstract
Purpose
Invasive non-functional pituitary adenomas (NFPAs) constitute 35% of NFPAs. Despite a relatively large body of molecular investigations on the invasiveness of NFPA, the underlying molecular mechanisms of invasiveness are yet to be determined. Herein, we aimed to provide an overview of gene/microRNA(miRNAs) expression alterations in invasive NFPA.
Methods
This article describes a systematic literature review of articles published up to March 23, 2021, on the transcriptional alterations of invasive NFPA. Five digital libraries were searched, and 42 articles in total fulfilled the eligibility criteria. Pathway enrichment was conducted, and protein interactions among the identified deregulated genes were inferred.
Results
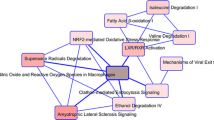
In total 133 gene/protein transcriptional alterations, comprising 87 increased and 46 decreased expressions, were detected in a collective number of 1001 invasive compared with 1007 non-invasive patients with NFPA. Deregulation of CDH1, PTTG1, CCNB1, SNAI1, SLUG, EZR, and PRKACB, which are associated with epidermal-mesenchymal transition (EMT), was identified. Moreover, six members of the angiogenesis pathway, i.e., VEGFA, FLT1, CCND1, CTNNB1, MYC(c-MYC), and PTTG1, were detected. SLC2A1, FLT1, and VEGFA were also recognized in the hypoxia pathway. Physical interactions of CTNNB1 with FLT1, CCND1, and EZR as well as its indirect interactions with VEGFA, MYC, CCNB1, and PCNA indicate the tight interplay between EMT, angiogenesis, and hypoxia pathways in invasive NFPAs. In addition, Hippo, JAK-STAT, MAPK, Wnt, PI3K-Akt, Ras, TGF-b, VEGF, and ErbB were identified as interwoven signaling pathways.
Conclusion
In conclusion, invasive NFPA shares very common deregulated signaling pathways with invasive cancers. A large amount of heterogeneity in the reported deregulations in different studies necessitates the validation of the expressional changes of the suggested biomarkers in a large number of patients with invasive NFPA.





Similar content being viewed by others
Data availability
All articles used in this systematic review are provided in Supplementary Table 1.
References
M. Gruppetta, C. Mercieca, J. Vassallo, Prevalence and incidence of pituitary adenomas: a population based study in Malta. Pituitary 16(4), 545–553 (2013)
G. Ntali, J.A. Wass, Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary 21(2), 111–118 (2018)
P.U. Freda, J.N. Bruce, A.G. Khandji, Z. Jin, R.A. Hickman, E. Frey et al. Presenting features in 269 patients with clinically nonfunctioning pituitary adenomas enrolled in a prospective study. J. Endocr. Soc. 4(4), bvaa021 (2020)
E. Manojlovic-Gacic, B.E. Engström, O. Casar-Borota, Histopathological classification of non-functioning pituitary neuroendocrine tumors. Pituitary 21(2), 119–129 (2018)
A.S. Micko, A. Wöhrer, S. Wolfsberger, E. Knosp, Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J. Neurosurg. 122(4), 803–811 (2015)
J. Maletkovic, A. Dabbagh, D. Zhang, A. Zahid, M. Bergsneider, M.B. Wang et al. Residual Tumor Confers a 10-Fold Increased Risk of Regrowth in Clinically Nonfunctioning Pituitary Tumors. J. Endocr. Soc. 3(10), 1931–1941 (2019)
P.D. Delgado-López, J. Pi-Barrio, M.T. Dueñas-Polo, M. Pascual-Llorente, M.C. Gordón-Bolaños, Recurrent non-functioning pituitary adenomas: a review on the new pathological classification, management guidelines and treatment options. Clin. Transl. Oncol. 20(10), 1233–1245 (2018)
M.T. Walsh, W.T. Couldwell, Symptomatic cystic degeneration of a clinically silent corticotroph tumor of the pituitary gland. Skull Base 20(5), 367–370 (2010)
J. Trouillas, P. Roy, N. Sturm, E. Dantony, C. Cortet-Rudelli, G. Viennet et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathologica 126(1), 123–135 (2013)
S. Asioli, A. Righi, M. Iommi, C. Baldovini, F. Ambrosi, F. Guaraldi et al. Validation of a clinicopathological score for the prediction of post-surgical evolution of pituitary adenoma: retrospective analysis on 566 patients from a tertiary care centre. Eur. J. Endocrinol. 180(2), 127–34. (2019)
O. Mete, S. Ezzat, S.L. Asa, Biomarkers of aggressive pituitary adenomas. J. Mol. Endocrinol. 49(2), R69–R78 (2012)
Q. Yang, X. Li, Molecular Network Basis of Invasive Pituitary Adenoma: A Review. Front Endocrinol. 10, 7 (2019)
Y. Chen, H.-L. Chuan, S.-Y. Yu, C.-Z. Li, Z.-B. Wu, G.-L. Li et al. A novel invasive-related biomarker in three subtypes of nonfunctioning pituitary adenomas. World Neurosurg. 100, 514–21. (2017)
W.E. Farrell, D.J. Simpson, J.E. Bicknell, A.J. Talbot, A.S. Bates, R.N. Clayton, Chromosome 9p deletions in invasive and noninvasive nonfunctional pituitary adenomas: the deleted region involves markers outside of the MTS1 and MTS2 genes. Cancer Res. 57(13), 2703–2709 (1997)
R.V. Lloyd, B.W. Scheithauer, T. Kuroki, S. Vidal, K. Kovacs, L. Stefaneanu, Vascular endothelial growth factor (VEGF) expression in human pituitary adenomas and carcinomas. Endocr. Pathol. 10(3), 229–35. (1999)
C. McCabe, J. Khaira, K. Boelaert, A. Heaney, L. Tannahill, S. Hussain et al. Expression of pituitary tumour transforming gene (PTTG) and fibroblast growth factor‐2 (FGF‐2) in human pituitary adenomas: relationships to clinical tumour behaviour. Clin. Endocrinol. 58(2), 141–150 (2003)
X. Zhan, D.M. Desiderio, The use of variations in proteomes to predict, prevent, and personalize treatment for clinically nonfunctional pituitary adenomas. EPMA J. 1(3), 439–459 (2010)
L. Hood, Q. Tian, Systems approaches to biology and disease enable translational systems medicine. Genomics, Proteom. Bioinforma. 10(4), 181–185 (2012)
D.L. Longo, Tumor heterogeneity and personalized medicine. N. Engl. J. Med. 366(10), 956–957 (2012)
J.E. Bradner, D. Hnisz, R.A. Young, Transcriptional addiction in cancer. Cell 168(4), 629–43. (2017)
J. O’Brien, H. Hayder, Y. Zayed, C. Peng, Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 9, 402 (2018)
Y. Peng, C.M. Croce, The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 1(1), 1–9 (2016)
M.V. Kuleshov, M.R. Jones, A.D. Rouillard, N.F. Fernandez, Q. Duan, Z. Wang et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44(W1), W90–W7. (2016)
D. Szklarczyk, A.L. Gable, D. Lyon, A. Junge, S. Wyder, J. Huerta-Cepas et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47(D1), D607–D13. (2019)
H. Han, H. Shim, D. Shin, J.E. Shim, Y. Ko, J. Shin et al. TRRUST: a reference database of human transcriptional regulatory interactions. Sci. Rep. 5(1), 1–11 (2015)
Y. Chen, X. Wang, miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 48(D1), D127–D131 (2020)
M. Sato, R. Tamura, H. Tamura, T. Mase, K. Kosugi, Y. Morimoto et al. Analysis of tumor angiogenesis and immune microenvironment in non-functional pituitary endocrine tumors. J. Clin. Med. 8(5), 695 (2019)
S. Uraki, H. Ariyasu, K. Takeshima, S. Morita, H. Inaba, H. Furuta et al. MSH6/2 and PD-L1 expressions are associated with tumor growth and invasiveness in silent pituitary adenoma subtypes. Int. J. Mol. Sci. 21(8), 2831 (2020)
W. Jia, J. Zhu, T.A. Martin, A. Jiang, A.J. Sanders, W.G. Jiang, Epithelial-mesenchymal transition (EMT) markers in human pituitary adenomas indicate a clinical course. Anticancer Res. 35(5), 2635–2643 (2015)
Y.H. Kim, J.H. Kim, Transcriptome analysis identifies an attenuated local immune response in invasive nonfunctioning pituitary adenomas. Endocrinol. Metab. 34(3), 314–322 (2019)
P. Matoušek, P. Buzrla, Š. Reguli, J. Krajča, J. Dvořáčková, R. Lipina, Factors that predict the growth of residual nonfunctional pituitary adenomas: correlations between relapse and cell cycle markers. BioMed Res. Int. 2018, 1–9 (2018)
W. Saeger, B. Lüdecke, D. Lüdecke, Clinical tumor growth and comparison with proliferation markers in non-functioning (inactive) pituitary adenomas. Exp. Clin. Endocrinol. Diabetes 116(02), 80–85 (2008)
X. Wang, Y. Fang, Y. Zhou, X. Guo, K. Xu, C. Li, et al. SDF-1α/MicroRNA-134 axis regulates nonfunctioning pituitary neuroendocrine tumor growth via targeting VEGFA. Front. Endocrinol. 952, 1–10 (2020)
G. Trott, B.R. Ongaratti, C.B. de Oliveira Silva, G.D. Abech, T. Haag, C.G.S.L. Rech et al. PTTG overexpression in non-functioning pituitary adenomas: Correlation with invasiveness, female gender and younger age. Ann. Diagnostic Pathol. 41, 83–89 (2019)
L. Zhenye, L. Chuzhong, W. Youtu, L. Xiaolei, C. Lei, H. Lichuan et al. The expression of TGF-β1, Smad3, phospho-Smad3 and Smad7 is correlated with the development and invasion of nonfunctioning pituitary adenomas. J. Transl. Med. 12(1), 1–8 (2014)
M. Ghadir, M.E. Khamseh, M. Panahi-Shamsabad, M. Ghorbani, H. Akbari, A.Z. Mehrjardi et al. Cell proliferation, apoptosis, and angiogenesis in non-functional pituitary adenoma: association with tumor invasiveness. Endocrine 69(3), 596–603 (2020)
X. Liu, S. Ma, Y. Yao, G. Li, M. Feng, K. Deng et al. Differential expression of folate receptor alpha in pituitary adenomas and its relationship to tumor behavior. Neurosurgery 70(5), 1274–1280 (2012)
D. Zhemin, L. Bing, W. Qing, Yifeng M., Xiaojie L. Increase in Folate Receptor Alpha Expression in Nonfunctional Pituitary Adenomas. Turkish Neurosurgery. 25(2), 298–304 (2015)
C. Ling, M. Pease, L. Shi, V. Punj, M.S. Shiroishi, D. Commins et al. A pilot genome-scale profiling of DNA methylation in sporadic pituitary macroadenomas: association with tumor invasion and histopathological subtype. PLoS One 9(4), e96178 (2014)
S. Cheng, C. Li, W. Xie, Y. Miao, J. Guo, J. Wang et al. Integrated analysis of DNA methylation and mRNA expression profiles to identify key genes involved in the regrowth of clinically non-functioning pituitary adenoma. Aging (Albany NY) 12(3), 2408 (2020)
S. Cheng, W. Xie, Y. Miao, J. Guo, J. Wang, C. Li et al. Identification of key genes in invasive clinically non-functioning pituitary adenoma by integrating analysis of DNA methylation and mRNA expression profiles. J. Transl. Med. 17(1), 1–12 (2019)
H. Wang, K. Chen, Z. Yang, W. Li, C. Wang, G. Zhang et al. Diagnosis of Invasive Nonfunctional Pituitary Adenomas by Serum Extracellular Vesicles. Anal. Chem. 91(15), 9580–9589 (2019)
R. Mjelle, S.O. Dima, N. Bacalbasa, K. Chawla, A. Sorop, D. Cucu et al. Comprehensive transcriptomic analyses of tissue, serum, and serum exosomes from hepatocellular carcinoma patients. BMC Cancer 19(1), 1–13. (2019)
L. Zhang, W. Li, L. Cao, J. Xu, Y. Qian, H. Chen et al. PKNOX2 suppresses gastric cancer through the transcriptional activation of IGFBP5 and p53. Oncogene 38(23), 4590–4604 (2019)
J. Wang, N. Ding, Y. Li, H. Cheng, D. Wang, Q. Yang et al. Insulin-like growth factor binding protein 5 (IGFBP5) functions as a tumor suppressor in human melanoma cells. Oncotarget 6(24), 20636 (2015)
J.R. Hwang, Y.-J. Cho, Y. Lee, Y. Park, H.D. Han, H.J. Ahn et al. The C-terminus of IGFBP-5 suppresses tumor growth by inhibiting angiogenesis. Sci. Rep. 6(1), 1–12 (2016)
F. Galland, L. Lacroix, P. Saulnier, P. Dessen, G. Meduri, M. Bernier et al. Differential gene expression profiles of invasive and non-invasive non-functioning pituitary adenomas based on microarray analysis. Endocr. Relat. Cancer 17(2), 361–371 (2010)
B. Li, H.B. Zhu, G.D. Song, J.H. Cheng, C.Z. Li, Y.Z. Zhang et al. Regulating the CCNB1 gene can affect cell proliferation and apoptosis in pituitary adenomas and activate epithelial‑to‑mesenchymal transition. Oncol. Lett. 18(5), 4651–4658 (2019)
P. Zhao, P. Zhang, W. Hu, H. Wang, G. Yu, Z. Wang et al. Upregulation of cyclin B1 plays potential roles in the invasiveness of pituitary adenomas. J. Clin. Neurosci. 43, 267–73. (2017)
J. Gil, M. Jordà, B. Soldevila, M. Puig-Domingo, Epithelial–Mesenchymal Transition in the Resistance to Somatostatin Receptor Ligands in Acromegaly. Front. Endocrinol. 12, 226 (2021)
Y. Song, X. Ma, M. Zhang, M. Wang, G. Wang, Y. Ye et al. Ezrin Mediates Invasion and Metastasis in Tumorigenesis: A Review. Front. Cell Dev. Biol. 8, 1321 (2020)
N. Li, J. Kong, Z. Lin, Y. Yang, T. Jin, M. Xu et al. Ezrin promotes breast cancer progression by modulating AKT signals. Br. J. Cancer 120(7), 703–13. (2019)
C.M. Falch, A.Y. Sundaram, K.A. Øystese, K.R. Normann, T. Lekva, I. Silamikelis et al. Gene expression profiling of fast-and slow-growing non-functioning gonadotroph pituitary adenomas. Eur. J. Endocrinol. 178(3), 295–307 (2018)
M. Kanehisa, Y. Sato, M. Kawashima, M. Furumichi, M. Tanabe, KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44(D1), D457–D62. (2016)
A.S. Farrell, R.C. Sears, MYC degradation. Cold Spring Harb. Perspect. Med. 4(3), a014365 (2014)
C. McCabe, K. Boelaert, L. Tannahill, A. Heaney, A. Stratford, J. Khaira et al. Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumors. J. Clin. Endocrinol. Metab. 87(9), 4238–4244 (2002)
Y. Li, L.-P. Zhou, P. Ma, C.-G. Sui, F.-D. Meng, X. Tian et al. Relationship of PTTG expression with tumor invasiveness and microvessel density of pituitary adenomas: a meta-analysis. Genet. Test. Mol. Biomark. 18(4), 279–285 (2014)
W. Zhou, Y. Song, H. Xu, K. Zhou, W. Zhang, J. Chen et al. In nonfunctional pituitary adenomas, estrogen receptors and slug contribute to development of invasiveness. J. Clin. Endocrinol. Metab. 96(8), E1237–E45. (2011)
A.P. Heaney, G.A. Horwitz, Z. Wang, R. Singson, S. Melmed, Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat. Med. 5(11), 1317–1321 (1999)
Y. Qian, C. Zhang, W. Wang, D. Lu, J. Li, L. Li et al. Hypoxia promotes proliferation of pituitary adenomas by HIF-1α/ALKBH5 signaling in vitro. Int. J. Clin. Exp. Pathol. 13(5), 1030 (2020)
S.-Y. Yu, L.-C. Hong, J. Feng, Y.-T. Wu, Y.-Z. Zhang, Integrative proteomics and transcriptomics identify novel invasive-related biomarkers of non-functioning pituitary adenomas. Tumor Biol. 37(7), 8923–8930 (2016)
J. Guo, Q. Fang, Y. Liu, W. Xie, Y. Zhang, C. Li, Identifying critical protein‑coding genes and long non‑coding RNAs in non‑functioning pituitary adenoma recurrence. Oncol. Lett. 21(4), 1- (2021)
J. Sinsky, K. Pichlerova, J. Hanes, Tau Protein Interaction Partners and Their Roles in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 22(17), 9207 (2021)
Z. Zimu, Z. Jia, F. Xian, M. Rui, R. Yuting, W. Yuan, et al. Decreased Expression of PACSIN1 in Brain Glioma Samples Predicts Poor Prognosis. Front. Mol. Biosci. 772, 1–11 (2021)
Z. Wei, C. Zhou, M. Li, R. Huang, H. Deng, S. Shen et al. Integrated multi-omics profiling of nonfunctioning pituitary adenomas. Pituitary 24(3), 312–25 (2021)
J. Boresowicz, P. Kober, N. Rusetska, M. Maksymowicz, A. Paziewska, M. Dąbrowska, et al. The Search of miRNA Related to Invasive Growth of Nonfunctioning Gonadotropic Pituitary Tumors. Int. J. Endocrinol. 2020, 1–8 (2020)
L. Chen, Y. Liu, Y. Hou, Y. Kato, H. Sano, T. Kanno, Expression and structure of interleukin 4 receptor (IL-4R) complex in human invasive pituitary adenomas. Neurosci. Lett. 417(1), 30–35 (2007)
S.J. Park, H. Kim, S.H. Kim, E.-H. Joe, I. Jou, Epigenetic downregulation of STAT6 increases HIF-1α expression via mTOR/S6K/S6, leading to enhanced hypoxic viability of glioma cells. Acta Neuropathologica Commun. 7(1), 1–19 (2019)
Y. Wu, J. Bai, L. Hong, C. Liu, S. Yu, G. Yu et al. Low expression of secreted frizzled-related protein 2 and nuclear accumulation of β-catenin in aggressive nonfunctioning pituitary adenoma. Oncol. Lett. 12(1), 199–206 (2016)
S. Yu, X. Wang, K. Cao, X. Bao, J. Yu, Identification of CDK6 and RHOU in serum exosome as biomarkers for the invasiveness of non-functioning pituitary adenoma. Chin. Med. Sci. J. 34(3), 168–176 (2019)
V. Cescato, E. Pinto, N. de Castro Musolino, S. Siqueira, M. Teixeira, editors. BCL2, BAX and CASP3 Apoptosis Related Genes Expression in Non-Functioning Pituitary Adenoma and Their Role as Potential Markers of Tumor Behavior. Endocr. Rev. 240–241 (2010)
M. Malumbres, M. Barbacid, Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9(3), 153–66. (2009)
T. Komori, Regulation of Rb family proteins by Cdk6/Ccnd1 in growth plates. Cell Cycle 12(14), 2161–2162 (2013)
L.J.T. de Araújo, A.M. Lerario, M. de Castro, C.S. Martins, M.D. Bronstein, M.C. Machado et al. Transcriptome analysis showed a differential signature between invasive and non-invasive corticotrophinomas. Front. Endocrinol. 8, 55 (2017)
S. Melmed, Pathogenesis of pituitary tumors. Nat. Rev. Endocrinol. 7(5), 257–266 (2011)
N. Sun, A. Taguchi, S. Hanash, Switching roles of TGF-β in cancer development: implications for therapeutic target and biomarker studies. J. Clin. Med. 5(12), 109 (2016)
S. Espiard, M.J. Knape, K. Bathon, G. Assié, M. Rizk-Rabin, S. Faillot, et al. Activating PRKACB somatic mutation in cortisol-producing adenomas. JCI insight. 3(8), 1–11 (2018)
Acknowledgements
The authors gratefully acknowledge the support provided by the Iran University of Medical Sciences.
Author contributions
M.K. designed and supervised the project. Z.E. conducted a literature search. N.H., M.H., S.C., and N.H.M. performed data extraction from the selected articles. N.H. carried out network analysis and wrote the manuscript. M.K. reviewed the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Hosseinkhan, N., Honardoost, M., Emami, Z. et al. A systematic review of molecular alterations in invasive non-functioning pituitary adenoma. Endocrine 77, 500–509 (2022). https://doi.org/10.1007/s12020-022-03105-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03105-9