Abstract
Objective
Patients with adrenal insufficiency have difficulties in fasting during the month of Ramadan with an increased risk of complications. Cortisol levels are unknown in these patients. The objective of this study was to assess the daily cortisol profile in hydrocortisone-treated patients with secondary adrenal insufficiency (SAI) and healthy controls during a fasting day.
Methods
A cross-sectional matched case-control study on 50 hydrocortisone-treated SAI patients and 69 controls who are used to fast. Clinical and therapeutic data were collected. Five salivary samples for cortisol measurement were collected throughout a fasting day of the third week of Ramadan 2019.
Results
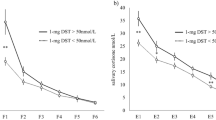
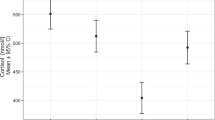
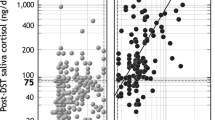
Salivary cortisol levels were significantly higher on awakening, at midnight and before the predawn meal in patients compared with controls. The circadian cortisol rhythm was disrupted in patients. The area under the salivary cortisol level versus time curve (AUC) was lower than the 2.5th percentile of the controls in one patient (2.5%) and higher than the 97.5th percentile in 23 patients (59%) who were considered overtreated. Age ≥ 35 years was independently associated with overtreatment (adjusted odds ratio = 12.0; 95% CI (2.0–70.4); p = 0.006). Seven patients broke their fasting for a complication compared with no one of the controls (p = 0.001). No factor was associated with this risk.
Conclusions
Salivary cortisol levels were high in fasting hydrocortisone-treated SAI patients with a disruption of the circadian rhythm.

Similar content being viewed by others
References
J.W. Buning, D.J. Touw, P. Brummelman, R.P.F. Dullaart, G. Van Den Berg, et al., Pharmacokinetics of oral hydrocortisone—results and implications from a randomized controlled trial. Metabolism (2017). https://doi.org/10.1016/j.metabol.2017.02.005
A. Oprea, N.C.G. Bonnet, O. Pollé, Ph. A. Lysy, Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther. Adv. Endocrinol. Metab. (2019). https://doi.org/10.1177/2042018818821294
M. Debono, Fasting during the Ramadan: a challenge for patients with adrenal insufficiency. Endocrine (2017). https://doi.org/10.1007/s12020-017-1329-y
M. Chihaoui, F. Chaker, M. Yazidi, W. Grira, Z. Ben Amor, et al., Ramadan fasting in patients with adrenal insufficiency. Endocrine (2017). https://doi.org/10.1007/s12020-016-1186-0
M. Chihaoui, W. Grira, J. Bettaieb, M. Yazidi, F. Chaker, et al., The risk for hypoglycemia during Ramadan fasting in patients with adrenal insufficiency. Nutrition (2018). https://doi.org/10.1016/j.nut.2017.07.014
M. Haouari, F. Haouari-Oukerro, A. Sfaxi, M.C. Ben Rayana, N. Kâabachi, et al., How Ramadan fasting affects caloric consumption, body weight, and circadian evolution of cortisol serum levels in young, healthy male volunteers. Horm. Metab. Res. (2008). https://doi.org/10.1055/s-2008-1065321
L. Ben Salem, S. Bchir, F. Bchir, R. Bouguerra, C. Ben Slama, Circadian rhythm of cortisol and its responsiveness to ACTH during Ramadan. Ann. Endocrinol. 63, 497–501 (2002)
S. Bahijri, A. Borai, G. Ajabnoor, A. Abdul Khaliq, I. AlQassas, et al., Relative metabolic stability, but disrupted circadian cortisol secretion during the fasting month of Ramadan. PLoS ONE (2013). https://doi.org/10.1371/journal.pone.0060917
C. Jung, S. Greco, H.H. Nguyen, J.T. Ho, J.G. Lewis, et al., Plasma, salivary and urinary cortisol levels following physiological and stress doses of hydrocortisone in normal volunteers. BMC Endocr. Disord. (2014). https://doi.org/10.1186/1472-6823-14-91
J.G. Lewis, Steroid analysis in saliva: an overview. Clin. Biochem. Rev. 27, 139–146 (2006)
M. Mezzullo, F. Fanelli, A. Fazzini, A. Gambineri, V. Vicennati, et al., Validation of an LC-MS/MS salivary assay for glucocorticoid status assessment: evaluation of the diurnal fluctuation of cortisol and cortisone and of their association within and between serum and saliva. J. Steroid Biochem. Mol. Biol. (2016). https://doi.org/10.1016/j.jsbmb.2016.04.012
K. Lovas, T.E. Thorsen, E.S. Husebye, Saliva cortisol measurement: simple and reliable assessment of the glucocorticoid replacement therapy in Addison’s disease. J. Endocrinol. Investig. (2006). https://doi.org/10.1007/BF03344183
F. Ceccato, N. Albiger, G. Reimondo, A. Chiara Frigo, S. Ferasin, et al., Assessment of glucocorticoid therapy with salivary cortisol in secondary adrenal insufficiency. Eur. J. Endocrinol. (2012). https://doi.org/10.1530/eje-12-0534
L. Smans, E. Lentjes, A. Hermus, P. Zelissen, Salivary cortisol day curves in assessing glucocorticoid replacement therapy in Addison’s disease. Hormones (2013). https://doi.org/10.1007/BF03401290
H. Raff, R.J. Singh, Measurement of late-night salivary cortisol and cortisone by LC-MS/MS to assess pre analytical sample contamination with topical hydrocortisone. Clin. Chem. (2012). https://doi.org/10.1373/clinchem.2012.182717
M. Debono, R.F. Harrison, M.J. Whitaker, D. Eckland, W. Arlt, et al., Salivary cortisone reflects cortisol exposure under physiological conditions and after hydrocortisone. J. Clin. Endocrinol. Metab. (2016). https://doi.org/10.1210/jc.2015-3694
S. Tunn, H. Mollmann, J. Barth, H. Derendorf, M. Krieg, Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration. Clin. Chem. 38, 1491–1494 (1992)
E. Aardal, A.C. Holm, Cortisol in saliva–reference ranges and relation to cortisol in serum. Eur. J. Clin. Chem. Clin. Biochem. (1995). https://doi.org/10.1515/cclm.1995.33.12.927
D. Czock, F. Keller, F.M. Rasche, et al., Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. (2005). https://doi.org/10.2165/00003088-200544010-00003
E. Charmandari, A. Johnston, C.G.D. Brook and P.C. Hindmarsh. Bioavailability of oral hydrocortisone in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Endocrinol. (2001). https://doi.org/10.1677/joe.0.1690065
H. Derendorf, H. Möllmann, J. Barth, C. Möllmann, S. Tunn, et al., Pharmacokinetics and oral bioavailability of hydrocortisone. J. Clin. Pharmacol. (1991). https://doi.org/10.1002/j.1552-4604.1991.tb01906.x
I. Perogamvros, L.J. Owen, J. Newell-Price, D.W. Ray, P.J. Trainer, Simultaneous measurement of cortisol and cortisone in human saliva using liquid chromatography-tandem mass spectrometry: application in basal and stimulated conditions. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. (2009). https://doi.org/10.1016/j.jchromb.2009.09.014
E. Rousseau, M. Joubert, G. Trzepla, J.J. Parienti, T. Freret, et al., Usefulness of time-point serum cortisol and ACTH measurements for the adjustment of glucocorticoid replacement in adrenal insufficiency. PLoS ONE (2015). https://doi.org/10.1371/journal.pone.0135975
P.M. Mah, R.C. Jenkins, A. Rostami-Hodjegan, J. Newell-Price, A. Doane, et al., Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin. Endocrinol. (2004). https://doi.org/10.1111/j.1365-2265.2004.02106.x
R. Miller, T. Stalder, M. Jarczok, D.M. Almeida, E. Badrick, The CIRCORT database: reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology (2016). https://doi.org/10.1016/j.psyneuen.2016.07.201
A.M. Maguire, G.R. Ambler, B. Moore, K. Waite, M. McLean, et al., The clinical utility of alternative, less invasive sampling techniques in the assessment of oral hydrocortisone therapy in children and adolescents with hypopituitarism. Eur. J. Endocrinol. (2007). https://doi.org/10.1530/EJE-06-0700
S. Dinneen, A. Alzaid, J. Miles, R. Rizza, Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J. Clin. Investig. (1993). https://doi.org/10.1172/JCI116832
Y.M. Jang, E.J. Lee, D.L. Kim, S.K. Kim, K.H. Song. The association between midnight salivary cortisol and metabolic syndrome in Korean adults. Diabetes Metab. J. (2012). https://doi.org/10.4093/dmj.2012.36.3.245
G.J. Mangos, J.A. Whitworth, P.M. Williamson, J.J. Kelly. Glucocorticoids and the kidney. Nephrology (2003). https://doi.org/10.1111/j.1440-1797.2003.00215.x
L.I. McKay, J.A. Cidlowski. Corticosteroids. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003. Chapter 62. https://www.ncbi.nlm.nih.gov/books/NBK12594/
F. Ceccato, M. Barbot, L. Lizzul, E. Selmin, A. Saller, Decrease in salivary cortisol levels after glucocorticoid dose reduction in patients with adrenal insufficiency: a prospective proof-of-concept study. Clin Endocrinol. (2018). https://doi.org/10.1111/cen.13490
D.W. Cain, J.A. Cidlowski, Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract Res Clin Endocrinol Metab. (2015). https://doi.org/10.1016/j.beem.2015.04.007
S. Ramamoorthy, J.A. Cidlowski, Corticosteroids mechanisms of action in health and disease. Rheum. Dis. Clin. N. Am. (2016). https://doi.org/10.1016/j.rdc.2015.08.002
K. Pierre, N. Schlesinger, and I. P. Androulakis. The hepato-hypothalamic-pituitary-adrenal-renal axis: mathematical modeling of cortisol’s production, metabolism, and seasonal variation. J. Biol. Rhythms. (2017). https://doi.org/10.1177/0748730772929
S. Diederich, E. Eigendorff, P. Burkhardt, M. Quinkler, C. Bumke-Vogt et al., 11beta-hydroxysteroid dehydrogenase types 1 and 2: an important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids. J. Clin. Endocrinol. Metab. (2002). https://doi.org/10.1210/jc.2002-020970
M.S. Cooper, P.M. Stewart, 11 beta hydroxysteroid dehydrogenase type 1 and its role in the hypothalamus-pituitary-adrenal axis, metabolic syndrome, and inflammation. J. Clin. Endocrinol. Metab. (2009). https://doi.org/10.1210/jc.2009-1412
A. Goecke, J. Guerrero. Glucocorticoid receptor beta in acute and chronic inflammatory conditions: clinical implications. Immunobiology (2006). https://doi.org/10.1016/j.imbio.2005.11.002
R. Miller, F. Plessow, M. Rauh, M. Gröschl, C. Kirschbaum. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology (2013). https://doi.org/10.1016/j.psyneuen.2012.04.019
M. Chihaoui, W. Mimita, I. Oueslati, O. Rejeb, Z. Ben Amor, et al., Prednisolone or hydrocortisone replacement in patients with corticotrope deficiency fasting during Ramadan result in similar risks of complications and quality of life: a randomized double-blind controlled trial. Endocrine (2020). https://doi.org/10.1007/s12020-019-02082-w
E.L. Williams, S. Choudhury, T. Tan, K. Meeran. Prednisolone replacement therapy mimics the circadian rhythm more closely than other glucocorticoids. J. Appl. Lab. Med. (2016). https://doi.org/10.1373/jalm.2016.020206
M. Debono, C. Ghobadi, A. Rostami-Hodjegan, H. Huatan, M.J. Campbell, et al., Modified-release hydrocortisone to provide circadian cortisol profiles. J. Clin. Endocrinol. Metab. (2009). https://doi.org/10.1210/jc.2008-2380
F. Ceccato, E. Selmin, Ch. Sabbadin, M. Dalla Costa, G. Antonelli, et al., Improved salivary cortisol rhythm with dual-release hydrocortisone. Endocr. Connect. (2018). https://doi.org/10.1530/EC-18-0257
L.M. Mongioì, R.A. Condorelli, F. Barbagallo, et al., Dual-release hydrocortisone for treatment of adrenal insufficiency: a systematic review. Endocrine 67, 507–515 (2020). https://doi.org/10.1007/s12020-020-02187-7
Acknowledgements
The authors thank Professor Bechir Zouari for his valuable guidance in the statistical analysis.
Funding
This research was founded by the University Hospital la Rabta, Tunis. We declare no financial relationship with any organization or industry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University Hospital la Rabta and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chihaoui, M., Madhi, W., Yazidi, M. et al. Salivary cortisol levels during Ramadan fasting in hydrocortisone-treated secondary adrenal insufficiency patients. Endocrine 70, 404–411 (2020). https://doi.org/10.1007/s12020-020-02452-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02452-9