Abstract
Human milk is a complex fluid that contains carbohydrates, lipids, proteins, and other bioactive molecules (immunoglobulins, lactoferrin, human milk oligosaccharides, lysozyme, leukocytes, cytokines, hormones, and microbiome) which provide nutritional, immunological, and developmental benefits to the infant. In addition to their involvement in the development, these bioactive compounds have a key role in anti-oncogenicity, neuro-cognitive development, cellular communication, and differentiation. As a result of technological advancements, it has been discovered that human breast milk contains cells that display many of the characteristics of stem cells with multilineage differentiation potentials. Do these cells have any specific properties or roles? Research efforts on breast milk cells have been mainly focused on leukocytes based on their immunological perspective in the early postpartum period. This review summarizes the nutritional components in human milk, i.e., the macro and micronutrients required for the growth and development of infants. Further, it discusses the research work reported concerning the purification, propagation, and differentiation of breast milk progenitor cells and highlights the advancements made in this newly emerging field of stem cell biology and regenerative medicine.
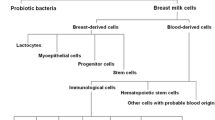
Graphical Abstract






Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Jenness, R. (1979). The composition of human milk. Seminars in Perinatology, 3(3), 225–239.
Kaingade, P., Somasundaram, I., Nikam, A., Behera, P., Kulkarni, S., & Patel, J. (2017). Breast milk cell components and its beneficial effects on neonates: Need for breast milk cell banking. Journal of Pediatric and Neonatal Individualized Medicine (JPNIM), 6(1), e060115–e060115. https://doi.org/10.7363/060115
Hassiotou, F., & Geddes, D. (2013). Anatomy of the human mammary gland: Current status of knowledge. Clinical Anatomy (New York, N.Y.), 26(1), 29–48. https://doi.org/10.1002/ca.22165
Key, T. J., Verkasalo, P. K., & Banks, E. (2001). Epidemiology of breast cancer. The Lancet. Oncology, 2(3), 133–140. https://doi.org/10.1016/S1470-2045(00)00254-0
Patki, S., Kadam, S., Chandra, V., & Bhonde, R. (2010). Human breast milk is a rich source of multipotent mesenchymal stem cells. Human Cell, 23(2), 35–40. https://doi.org/10.1111/j.1749-0774.2010.00083.x
World Health Organization. Division of Diarrhoeal and Acute Respiratory Disease Control, & Fund (UNICEF), U. N. C. (1993). Breastfeeding counselling : a training course (No. WHO/CDR/93.3–6). World Health Organization. Retrieved from https://apps.who.int/iris/handle/10665/63428. Accessed 19 May 2009
Sarhadi, N. S., Shaw Dunn, J., Lee, F. D., & Soutar, D. S. (1996). An anatomical study of the nerve supply of the breast, including the nipple and areola. British Journal of Plastic Surgery, 49(3), 156–164. https://doi.org/10.1016/s0007-1226(96)90218-0
Khan, Y. S., & Sajjad, H. (2022). Anatomy, Thorax, Mammary Gland. In StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK547666/. Accessed 25 July 2022
Anderson, S. M., Rudolph, M. C., McManaman, J. L., & Neville, M. C. (2007). Key stages in mammary gland development. Secretory activation in the mammary gland: it’s not just about milk protein synthesis! Breast Cancer Research, 9(1), 204. https://doi.org/10.1186/bcr1653.
Pillay, J., & Davis, T. J. (2022). Physiology, Lactation. In StatPearls. Treasure Island (FL): StatPearls Publishing. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK499981/. Accessed 18 July 2022
Neville, M. C., Morton, J., & Umemura, S. (2001). Lactogenesis. The transition from pregnancy to lactation. Pediatric Clinics of North America, 48(1), 35–52. https://doi.org/10.1016/s0031-3955(05)70284-4.
Neville, M. C., & Morton, J. (2001). Physiology and Endocrine Changes Underlying Human Lactogenesis II. The Journal of Nutrition, 131(11), 3005S-3008S. https://doi.org/10.1093/jn/131.11.3005S
Anderson, E., Clarke, R. B., & Howell, A. (1998). Estrogen responsiveness and control of normal human breast proliferation. Journal of Mammary Gland Biology and Neoplasia, 3(1), 23–35. https://doi.org/10.1023/a:1018718117113
Norman, A. W., & Henry, H. L. (2015). Chapter 14 - Hormones of Pregnancy, Parturition and Lactation. In A. W. Norman & H. L. Henry (Eds.), Hormones (Third Edition) (pp. 297–320). Academic Press. https://doi.org/10.1016/B978-0-08-091906-5.00014-8.
The physiological basis of breastfeeding. (2009). Infant and Young Child Feeding: Model Chapter for Textbooks for Medical Students and Allied Health Professionals. World Health Organization. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK148970/.
Ballard, O., & Morrow, A. L. (2013). Human Milk Composition: Nutrients and Bioactive Factors. Pediatric Clinics of North America, 60(1), 49–74. https://doi.org/10.1016/j.pcl.2012.10.002
Valentine, C. J., & Wagner, C. L. (2013). Nutritional Management of the Breastfeeding Dyad. Pediatric Clinics, 60(1), 261–274. https://doi.org/10.1016/j.pcl.2012.10.008
Eriksen, K. G., Christensen, S. H., Lind, M. V., & Michaelsen, K. F. (2018). Human milk composition and infant growth. Current Opinion in Clinical Nutrition and Metabolic Care, 21(3), 200–206. https://doi.org/10.1097/MCO.0000000000000466
Mosca, F., & Giannì, M. L. (2017). Human milk: Composition and health benefits. La Pediatria Medica E Chirurgica: Medical and Surgical Pediatrics, 39(2), 155. https://doi.org/10.4081/pmc.2017.155
Perrella, S., Gridneva, Z., Lai, C. T., Stinson, L., George, A., Bilston-John, S., & Geddes, D. (2021). Human milk composition promotes optimal infant growth, development and health. Seminars in Perinatology, 45(2), 151380. https://doi.org/10.1016/j.semperi.2020.151380.
Maas, Y. G. H., Gerritsen, J., Hart, A. A. M., Hadders-Algra, M., Ruijter, J. M., Tamminga, P., … Spekreijse, H. (1998). Development of macronutrient composition of very preterm human milk. British Journal of Nutrition, 80(1), 35–40. https://doi.org/10.1017/S0007114598001743.
Lönnerdal, B. (2004). Human milk proteins: Key components for the biological activity of human milk. Advances in Experimental Medicine and Biology, 554, 11–25.
Molinari, C. E., Casadio, Y. S., Hartmann, B. T., Livk, A., Bringans, S., Arthur, P. G., & Hartmann, P. E. (2012). Proteome mapping of human skim milk proteins in term and preterm milk. Journal of Proteome Research, 11(3), 1696–1714. https://doi.org/10.1021/pr2008797
Liao, Y., Alvarado, R., Phinney, B., & Lönnerdal, B. (2011). Proteomic characterization of human milk whey proteins during a twelve-month lactation period. Journal of Proteome Research, 10(4), 1746–1754. https://doi.org/10.1021/pr101028k
Lönnerdal, B. (2003). Nutritional and physiologic significance of human milk proteins. The American Journal of Clinical Nutrition, 77(6), 1537S-1543S. https://doi.org/10.1093/ajcn/77.6.1537S
Meurant: Handbook of milk composition - Google Scholar. (2022, June 22). Retrieved June 22, 2022, from https://scholar.google.com/scholar_lookup?hl=en&publication_year=1995&pages=115-221&author=A.+Prenticeauthor=R.G.+Jensens&title=Handbook+of+milk+composition.
Liao, Y., Weber, D., Xu, W., Durbin-Johnson, B. P., Phinney, B. S., & Lönnerdal, B. (2017). Absolute Quantification of Human Milk Caseins and the Whey/Casein Ratio during the First Year of Lactation. Journal of Proteome Research, 16(11), 4113–4121. https://doi.org/10.1021/acs.jproteome.7b00486
Grote, V., Verduci, E., Scaglioni, S., Vecchi, F., Contarini, G., Giovannini, M., … Agostoni, C. (2016). Breast milk composition and infant nutrient intakes during the first 12 months of life. European Journal of Clinical Nutrition, 70(2), 250–256. https://doi.org/10.1038/ejcn.2015.162.
Macronutrient and energy contents of human milk fractions during the first six months of lactation - PubMed. (2022, July 28). Retrieved July 28, 2022, from https://pubmed.ncbi.nlm.nih.gov/16203669/.
Presence of Leptin in Colostrum and/or Breast Milk from Lactating Mothers: A Potential Role in the Regulation of Neonatal Food Intake | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic. (2022, August 23). Retrieved August 23, 2022, from https://academic.oup.com/jcem/article/82/12/4270/2866121.
Guesnet, P., & Alessandri, J.-M. (2011). Docosahexaenoic acid (DHA) and the developing central nervous system (CNS) – Implications for dietary recommendations. Biochimie, 93(1), 7–12. https://doi.org/10.1016/j.biochi.2010.05.005
Kashyap, S., Towers, H. M., Sahni, R., Ohira-Kist, K., Abildskov, K., & Schulze, K. F. (2001). Effects of quality of energy on substrate oxidation in enterally fed, low-birth-weight infants. American Journal of Clinical Nutrition, 74(3), 374–380. https://doi.org/10.1093/ajcn/74.3.374
Kunz, C., Rodriguez-Palmero, M., Koletzko, B., & Jensen, R. (1999). Nutritional and biochemical properties of human milk, Part I: General aspects, proteins, and carbohydrates. Clinics in Perinatology, 26(2), 307–333.
Hester, S. N., Hustead, D. S., Mackey, A. D., Singhal, A., & Marriage, B. J. (2012). Is the macronutrient intake of formula-fed infants greater than breast-fed infants in early infancy? Journal of Nutrition and Metabolism, 2012, 891201. https://doi.org/10.1155/2012/891201.
Martin, C. R., Ling, P.-R., & Blackburn, G. L. (2016). Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients, 8(5), 279. https://doi.org/10.3390/nu8050279
Donovan, S. M., & Comstock, S. S. (2016). Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Annals of Nutrition & Metabolism, 69(Suppl 2), 42–51. https://doi.org/10.1159/000452818
Harmsen, H. J., Wildeboer-Veloo, A. C., Raangs, G. C., Wagendorp, A. A., Klijn, N., Bindels, J. G., & Welling, G. W. (2000). Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. Journal of Pediatric Gastroenterology and Nutrition, 30(1), 61–67. https://doi.org/10.1097/00005176-200001000-00019
Plaza-Díaz, J., Fontana, L., & Gil, A. (2018). Human Milk Oligosaccharides and Immune System Development. Nutrients, 10(8), E1038. https://doi.org/10.3390/nu10081038
Greer, F. R. (2001). Do breastfed infants need supplemental vitamins? Pediatric Clinics of North America, 48(2), 415–423. https://doi.org/10.1016/s0031-3955(08)70034-8
Black, R. E., Allen, L. H., Bhutta, Z. A., Caulfield, L. E., de Onis, M., Ezzati, M., … Rivera, J. (2008). Maternal and child undernutrition: global and regional exposures and health consequences. The Lancet, 371(9608), 243–260. https://doi.org/10.1016/S0140-6736(07)61690-0.
Choi, Y. K., Kim, J.-M., Lee, J.-E., Cho, M. S., Kang, B. S., Choi, H., & Kim, Y. (2016). Association of Maternal Diet With Zinc, Copper, and Iron Concentrations in Transitional Human Milk Produced by Korean Mothers. Clinical Nutrition Research, 5(1), 15–25. https://doi.org/10.7762/cnr.2016.5.1.15
Allen, L. H. (2012). B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Advances in Nutrition (Bethesda, Md.), 3(3), 362–369. https://doi.org/10.3945/an.111.001172.
Dawodu, A., Zalla, L., Woo, J. G., Herbers, P. M., Davidson, B. S., Heubi, J. E., & Morrow, A. L. (2014). Heightened attention to supplementation is needed to improve the vitamin D status of breastfeeding mothers and infants when sunshine exposure is restricted. Maternal & Child Nutrition, 10(3), 383–397. https://doi.org/10.1111/j.1740-8709.2012.00422.x
Guideline: daily iron supplementation in infants and children. (2022, August 22). Retrieved August 22, 2022, from https://www.who.int/publications-detail-redirect/9789241549523.
Update on zinc deficiency and excess in clinical pediatric practice - PubMed. (2022, August 22). Retrieved August 22, 2022, from https://pubmed.ncbi.nlm.nih.gov/23689110/.
Minerals and Trace Elements in Human Breast Milk Are Associated with Guatemalan Infant Anthropometric Outcomes within the First 6 Months - PubMed. (2022, August 22). Retrieved August 22, 2022, from https://pubmed.ncbi.nlm.nih.gov/27558578/.
Morrow, A. L., Ruiz-Palacios, G. M., Jiang, X., & Newburg, D. S. (2005). Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. The Journal of Nutrition, 135(5), 1304–1307. https://doi.org/10.1093/jn/135.5.1304
Newburg, D. S., Ruiz-Palacios, G. M., & Morrow, A. L. (2005). Human milk glycans protect infants against enteric pathogens. Annual Review of Nutrition, 25, 37–58. https://doi.org/10.1146/annurev.nutr.25.050304.092553
Hunt, K. M., Foster, J. A., Forney, L. J., Schütte, U. M. E., Beck, D. L., Abdo, Z., … McGuire, M. A. (2011). Characterization of the Diversity and Temporal Stability of Bacterial Communities in Human Milk. PLOS ONE, 6(6), e21313. https://doi.org/10.1371/journal.pone.0021313.
The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery - PubMed. (2022, August 22). Retrieved August 22, 2022, from https://pubmed.ncbi.nlm.nih.gov/22836031/.
Shelby, R. D., Cromeens, B., Rager, T. M., & Besner, G. E. (2019). Influence of Growth Factors on the Development of NEC. Clinics in Perinatology, 46(1), 51–64. https://doi.org/10.1016/j.clp.2018.10.005
Lawrence, R. M., & Pane, C. A. (2007). Human breast milk: Current concepts of immunology and infectious diseases. Current Problems in Pediatric and Adolescent Health Care, 37(1), 7–36. https://doi.org/10.1016/j.cppeds.2006.10.002
Loui, A., Eilers, E., Strauss, E., Pohl-Schickinger, A., Obladen, M., & Koehne, P. (2012). Vascular Endothelial Growth Factor (VEGF) and soluble VEGF receptor 1 (sFlt-1) levels in early and mature human milk from mothers of preterm versus term infants. Journal of Human Lactation: Official Journal of International Lactation Consultant Association, 28(4), 522–528. https://doi.org/10.1177/0890334412447686
Levels of Growth Factors and IgA in the Colostrum of Women from Burundi and Italy - PMC. (2022, August 23). Retrieved August 23, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6164593/.
A Review of Bioactive Factors in Human Breastmilk: A Focus on Prematurity - PMC. (2022, August 23). Retrieved August 23, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6628333/.
Aydin, S., Aydin, S., Ozkan, Y., & Kumru, S. (2006). Ghrelin is present in human colostrum, transitional and mature milk. Peptides, 27(4), 878–882. https://doi.org/10.1016/j.peptides.2005.08.006
Baxter, R. C., Zaltsman, Z., & Turtle, J. R. (1984). Immunoreactive somatomedin-C/insulin-like growth factor I and its binding protein in human milk. The Journal of Clinical Endocrinology and Metabolism, 58(6), 955–959. https://doi.org/10.1210/jcem-58-6-955
Martin, L. J., Woo, J. G., Geraghty, S. R., Altaye, M., Davidson, B. S., Banach, W., … Morrow, A. L. (2006). Adiponectin is present in human milk and is associated with maternal factors. The American Journal of Clinical Nutrition, 83(5), 1106–1111. https://doi.org/10.1093/ajcn/83.5.1106
Ilcol, Y. O., Hizli, Z. B., & Eroz, E. (2008). Resistin is present in human breast milk and it correlates with maternal hormonal status and serum level of C-reactive protein. Clinical Chemistry and Laboratory Medicine, 46(1), 118–124. https://doi.org/10.1515/CCLM.2008.019
Update on breast milk hormones: leptin, ghrelin and adiponectin - PubMed. (2022, August 23). Retrieved August 23, 2022, from https://pubmed.ncbi.nlm.nih.gov/17950501/.
Bode, L., McGuire, M., Rodriguez, J. M., Geddes, D. T., Hassiotou, F., Hartmann, P. E., & McGuire, M. K. (2014). It’s Alive: Microbes and Cells in Human Milk and Their Potential Benefits to Mother and Infant123. Advances in Nutrition, 5(5), 571–573. https://doi.org/10.3945/an.114.006643
César, J. A., Victora, C. G., Barros, F. C., Santos, I. S., & Flores, J. A. (1999). Impact of breast feeding on admission for pneumonia during postneonatal period in Brazil: Nested case-control study. BMJ (Clinical Research Ed.), 318(7194), 1316–1320. https://doi.org/10.1136/bmj.318.7194.1316
Duijts, L., Ramadhani, M. K., & Moll, H. A. (2009). Breastfeeding protects against infectious diseases during infancy in industrialized countries. A systematic review. Maternal & Child Nutrition, 5(3), 199–210. https://doi.org/10.1111/j.1740-8709.2008.00176.x.
Duncan, B., Ey, J., Holberg, C. J., Wright, A. L., Martinez, F. D., & Taussig, L. M. (1993). Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics, 91(5), 867–872.
Oddy, W. H., Holt, P. G., Sly, P. D., Read, A. W., Landau, L. I., Stanley, F. J., … Burton, P. R. (1999). Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ, 319(7213), 815–819. https://doi.org/10.1136/bmj.319.7213.815.
Saarinen, U. M. (1982). Prolonged breast feeding as prophylaxis for recurrent otitis media. Acta Paediatrica Scandinavica, 71(4), 567–571. https://doi.org/10.1111/j.1651-2227.1982.tb09476.x
Wright, A. L., Holberg, C. J., Martinez, F. D., Morgan, W. J., & Taussig, L. M. (1989). Breast feeding and lower respiratory tract illness in the first year of life. Group Health Medical Associates. BMJ (Clinical Research Ed.), 299(6705), 946–949. https://doi.org/10.1136/bmj.299.6705.946
Zhou, L., Yoshimura, Y., Huang, Y., Suzuki, R., Yokoyama, M., Okabe, M., & Shimamura, M. (2000). Two independent pathways of maternal cell transmission to offspring: Through placenta during pregnancy and by breast-feeding after birth. Immunology, 101(4), 570–580. https://doi.org/10.1046/j.1365-2567.2000.00144.x
Nyquist, S. K., Gao, P., Haining, T. K., Retchin, M. R., Golan, Y., Drake, R. S., Kolb, K., Mead, B. E., Ahituv, N., Martinez, M. E., Shalek, A. K., Berger, B., & Goods, B. A. (2022). Cellular and transcriptional diversity over the course of human lactation. Proceedings of the National Academy of Sciences, 119(15):e2121720119. https://doi.org/10.1073/pnas.2121720119
Department and Clinic of Endocrinology, Diabetology and Isotope Therapy, Faculty of Postgraduate Medical Training, Wroclaw Medical University, Wrocław, Poland, Zdrojewicz, Z., Herman, M., Faculty of Medicine, Wroclaw Medical University, Wrocław, Poland, Sałamacha, M., Faculty of Medicine, Wroclaw Medical University, Wrocław, Poland, … Faculty of Medicine, Wroclaw Medical University, Wrocław, Poland. (2017). Human milk – facts and myths. Pediatria i Medycyna Rodzinna, 13(1), 11–20. https://doi.org/10.15557/PiMR.2017.0001.
Goldman, A. S. (2000). Modulation of the Gastrointestinal Tract of Infants by Human Milk. Interfaces and Interactions. An Evolutionary Perspective. The Journal of Nutrition, 130(2), 426S-431S. https://doi.org/10.1093/jn/130.2.426S.
Zheng, Y., Corrêa-Silva, S., de Souza, E. C., Maria Rodrigues, R., da Fonseca, F. A. M., Gilio, A. E., … Palmeira, P. (2020). Macrophage profile and homing into breast milk in response to ongoing respiratory infections in the nursing infant. Cytokine, 129, 155045. https://doi.org/10.1016/j.cyto.2020.155045.
Witkowska-Zimny, M., & Kaminska-El-Hassan, E. (2017). Cells of human breast milk. Cellular & Molecular Biology Letters, 22, 11. https://doi.org/10.1186/s11658-017-0042-4
Fitzstevens, J. L., Smith, K. C., Hagadorn, J. I., Caimano, M. J., Matson, A. P., & Brownell, E. A. (2017). Systematic Review of the Human Milk Microbiota. Nutrition in Clinical Practice: Official Publication of the American Society for Parenteral and Enteral Nutrition, 32(3), 354–364. https://doi.org/10.1177/0884533616670150
Perez, P. F., Doré, J., Leclerc, M., Levenez, F., Benyacoub, J., Serrant, P., … Donnet-Hughes, A. (2007). Bacterial Imprinting of the Neonatal Immune System: Lessons From Maternal Cells? Pediatrics, 119(3), e724–e732. https://doi.org/10.1542/peds.2006-1649.
Moossavi, S., & Azad, M. B. (2020). Origins of human milk microbiota: New evidence and arising questions. Gut Microbes, 12(1), 1667722. https://doi.org/10.1080/19490976.2019.1667722
Martín, R., Langa, S., Reviriego, C., Jimínez, E., Marín, M. L., Xaus, J., … Rodríguez, J. M. (2003). Human milk is a source of lactic acid bacteria for the infant gut. The Journal of Pediatrics, 143(6), 754–758. https://doi.org/10.1016/j.jpeds.2003.09.028.
Langa, S., Maldonado-Barragán, A., Delgado, S., Martín, R., Martín, V., Jiménez, E., … Rodríguez, J. M. (2012). Characterization of Lactobacillus salivarius CECT 5713, a strain isolated from human milk: from genotype to phenotype. Applied Microbiology and Biotechnology, 94(5), 1279–1287. https://doi.org/10.1007/s00253-012-4032-1.
Stark, P. L., & Lee, A. (1982). The Microbial Ecology of the Large Bowel of Breastfed and Formula-fed Infants During the First Year of Life. Journal of Medical Microbiology, 15(2), 189–203. https://doi.org/10.1099/00222615-15-2-189
Heikkilä, M. P., & Saris, P. E. J. (2003). Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. Journal of Applied Microbiology, 95(3), 471–478. https://doi.org/10.1046/j.1365-2672.2003.02002.x
Soto, A., Martín, V., Jiménez, E., Mader, I., Rodríguez, J. M., & Fernández, L. (2014). Lactobacilli and bifidobacteria in human breast milk: Influence of antibiotherapy and other host and clinical factors. Journal of Pediatric Gastroenterology and Nutrition, 59(1), 78–88. https://doi.org/10.1097/MPG.0000000000000347
Martín, R., Jiménez, E., Olivares, M., Marín, M. L., Fernández, L., Xaus, J., & Rodríguez, J. M. (2006). Lactobacillus salivarius CECT 5713, a potential probiotic strain isolated from infant feces and breast milk of a mother-child pair. International Journal of Food Microbiology, 112(1), 35–43. https://doi.org/10.1016/j.ijfoodmicro.2006.06.011
Martín, R., Olivares, M., Marín, M. L., Fernández, L., Xaus, J., & Rodríguez, J. M. (2005). Probiotic Potential of 3 Lactobacilli Strains Isolated From Breast Milk. Journal of Human Lactation, 21(1), 8–17. https://doi.org/10.1177/0890334404272393
Martín, R., Jiménez, E., Olivares, M., Marín, M. L., Fernández, L., Xaus, J., & Rodríguez, J. M. (2006). Lactobacillus salivarius CECT 5713, a potential probiotic strain isolated from infant feces and breast milk of a mother–child pair. International Journal of Food Microbiology, 112(1), 35–43. https://doi.org/10.1016/j.ijfoodmicro.2006.06.011
Lokesh, D., Parkesh, R., & Kammara, R. (2018). Bifidobacterium adolescentis is intrinsically resistant to antitubercular drugs. Scientific Reports, 8, 11897.https://doi.org/10.1038/s41598-018-30429-2.
Moubareck, C., Gavini, F., Vaugien, L., Butel, M. J., & Doucet-Populaire, F. (2005). Antimicrobial susceptibility of bifidobacteria. The Journal of Antimicrobial Chemotherapy, 55(1), 38–44. https://doi.org/10.1093/jac/dkh495
Martín, R., Jiménez, E., Heilig, H., Fernández, L., Marín, M. L., Zoetendal, E. G., & Rodríguez, J. M. (2009). Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Applied and Environmental Microbiology, 75(4), 965–969. https://doi.org/10.1128/AEM.02063-08
Campedelli, I., Mathur, H., Salvetti, E., Clarke, S., Rea, M. C., Torriani, S., … O’Toole, P. W. (2018). Genus-Wide Assessment of Antibiotic Resistance in Lactobacillus spp. Applied and Environmental Microbiology, 85(1), e01738–18. https://doi.org/10.1128/AEM.01738-18.
Anisimova, E. A., & Yarullina, D. R. (2019). Antibiotic Resistance of LACTOBACILLUS Strains. Current Microbiology, 76(12), 1407–1416. https://doi.org/10.1007/s00284-019-01769-7
Delgado, S., Flórez, A. B., & Mayo, B. (2005). Antibiotic susceptibility of Lactobacillus and Bifidobacterium species from the human gastrointestinal tract. Current Microbiology, 50(4), 202–207. https://doi.org/10.1007/s00284-004-4431-3
Olivares, M., Díaz-Ropero, M. P., Martín, R., Rodríguez, J. M., & Xaus, J. (2006). Antimicrobial potential of four Lactobacillus strains isolated from breast milk. Journal of Applied Microbiology, 101(1), 72–79. https://doi.org/10.1111/j.1365-2672.2006.02981.x
The commensal microflora of human milk: new perspectives for food bacteriotherapy and probiotics - ScienceDirect. (2022, August 30). Retrieved August 30, 2022, from https://www.sciencedirect.com/science/article/abs/pii/S0924224403002565.
Henrick, B. M., Yao, X.-D., Nasser, L., Roozrogousheh, A., & Rosenthal, K. L. (2017). Breastfeeding Behaviors and the Innate Immune System of Human Milk: Working Together to Protect Infants against Inflammation, HIV-1, and Other Infections. Frontiers in Immunology, 8, 1631. https://doi.org/10.3389/fimmu.2017.01631
Hassiotou, F., Hepworth, A. R., Metzger, P., Tat Lai, C., Trengove, N., Hartmann, P. E., & Filgueira, L. (2013). Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clinical & Translational Immunology, 2(4), e3. https://doi.org/10.1038/cti.2013.1.
Smith, C. W., & Goldman, A. S. (1970). Interactions of lymphocytes and macrophages from human colostrum: Characteristics of the interacting lymphocyte. Journal of the Reticuloendothelial Society, 8(1), 91–104.
Smith, C. W., & Goldman, A. S. (1968). The cells of human colostrum. I. In vitro studies of morphology and functions. Pediatric Research, 2(2), 103–109. https://doi.org/10.1203/00006450-196803000-00005.
Wold, A., & Adlerberth, I. (1998). Does breastfeeding affect the infant’s immune responsiveness? Acta Paediatrica, 87(1), 19–22. https://doi.org/10.1111/j.1651-2227.1998.tb01378.x
Holt, P. G., Oliver, J., Bilyk, N., McMenamin, C., McMenamin, P. G., Kraal, G., & Thepen, T. (1993). Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. The Journal of Experimental Medicine, 177(2), 397–407. https://doi.org/10.1084/jem.177.2.397
Jain, L., Vidyasagar, D., Xanthou, M., Ghai, V., Shimada, S., & Blend, M. (1989). In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Archives of Disease in Childhood, 64(7 Spec No), 930–933. https://doi.org/10.1136/adc.64.7_spec_no.930.
Papanicolaou, G. N., Bader, G. M., Holmquist, D. G., & Falk, E. A. (1956). Cytologic evaluation of breast secretions. Annals of the New York Academy of Sciences, 63(6), 1409–1421. https://doi.org/10.1111/j.1749-6632.1956.tb32146.x
Twigger, A.-J., Hepworth, A. R., Tat Lai, C., Chetwynd, E., Stuebe, A. M., Blancafort, P., … Kakulas, F. (2015). Gene expression in breastmilk cells is associated with maternal and infant characteristics. Scientific Reports, 5(1), 12933. https://doi.org/10.1038/srep12933.
Cregan, M. D., Fan, Y., Appelbee, A., Brown, M. L., Klopcic, B., Koppen, J., … Hartmann, P. E. (2007). Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell and Tissue Research, 329(1), 129–136. https://doi.org/10.1007/s00441-007-0390-x.
Mane, S., Taneja, S., Madala, J. S., Agarkhedkar, S., & Khetan, M. (2022). Study of Stem Cells in Human Milk. Cureus, 14(3). https://doi.org/10.7759/cureus.23701.
Hassiotou, F., Heath, B., Ocal, O., Filgueira, L., Geddes, D., Hartmann, P., & Wilkie, T. (2014). Breastmilk stem cell transfer from mother to neonatal organs (216.4). The FASEB Journal, 28(S1), 216.4. https://doi.org/10.1096/fasebj.28.1_supplement.216.4.
Ninkina, N., Kukharsky, M. S., Hewitt, M. V., Lysikova, E. A., Skuratovska, L. N., Deykin, A. V., & Buchman, V. L. (2019). Stem cells in human breast milk. Human Cell, 32(3), 223–230. https://doi.org/10.1007/s13577-019-00251-7
Kersin, S. G., & Özek, E. (2021). Breast milk stem cells: Are they magic bullets in neonatology? Turkish Archives of Pediatrics, 56(3), 187–191. https://doi.org/10.5152/TurkArchPediatr.2021.21006
Kinder, J. M., Jiang, T. T., Ertelt, J. M., Xin, L., Strong, B. S., Shaaban, A. F., & Way, S. S. (2015). Cross-Generational Reproductive Fitness Enforced by Microchimeric Maternal Cells. Cell, 162(3), 505–515. https://doi.org/10.1016/j.cell.2015.07.006
Full article: Long-term feto-maternal microchimerism revisited. (n.d.). https://doi.org/10.4161/chim.1.1.12743.
Immunological implications of pregnancy-induced microchimerism | Nature Reviews Immunology. (n.d.). Retrieved February 27, 2023, from https://www.nature.com/articles/nri.2017.38.
Breast milk MSCs: An explanation of tissue growth and maturation of offspring - Abd Allah - 2016 - IUBMB Life - Wiley Online Library. (n.d.). Retrieved February 27, 2023, from https://iubmb.onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/iub.1573.
Aydın, M. Ş, Yiğit, E. N., Vatandaşlar, E., Erdoğan, E., & Öztürk, G. (2018). Transfer and Integration of Breast Milk Stem Cells to the Brain of Suckling Pups. Scientific Reports, 8(1), 14289. https://doi.org/10.1038/s41598-018-32715-5
Breastfeeding-related maternal microchimerism | Nature Reviews Immunology. (n.d.). Retrieved February 27, 2023, from https://www.nature.com/articles/nri.2017.115.
Breastmilk cell trafficking induces microchimerism‐mediated immune system maturation in the infant - Molès - 2018 - Pediatric Allergy and Immunology - Wiley Online Library. (n.d.). Retrieved February 27, 2023, from https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/pai.12841.
Zhang, L., van Bree, S., van Rood, J. J., & Claas, F. H. (1991). Influence of breast feeding on the cytotoxic T cell allorepertoire in man. Transplantation, 52(5), 914–916.
Hanson, L. (2000). The mother-offspring dyad and the immune system. Acta Paediatrica, 89(3), 252–258. https://doi.org/10.1111/j.1651-2227.2000.tb01325.x
Ylostalo, J. H. (2021). 3D Stem Cell Culture. Retrieved from https://directory.doabooks.org/handle/20.500.12854/68280.
Khan, F. A., Almohazey, D., Alomari, M., & Almofty, S. A. (2018). Isolation, Culture, and Functional Characterization of Human Embryonic Stem Cells: Current Trends and Challenges. Stem Cells International, 2018, e1429351. https://doi.org/10.1155/2018/1429351.
McCulloch, E. A., & Till, J. E. (2005). Perspectives on the properties of stem cells. Nature Medicine, 11(10), 1026–1028. https://doi.org/10.1038/nm1005-1026
Mayhall, E. A., Paffett-Lugassy, N., & Zon, L. I. (2004). The clinical potential of stem cells. Current Opinion in Cell Biology, 16(6), 713–720. https://doi.org/10.1016/j.ceb.2004.09.007
Brown, C., McKee, C., Bakshi, S., Walker, K., Hakman, E., Halassy, S., … Chaudhry, G. R. (2019). Mesenchymal stem cells: Cell therapy and regeneration potential. Journal of Tissue Engineering and Regenerative Medicine, 13(9), 1738–1755. https://doi.org/10.1002/term.2914
Bacakova, L., Zarubova, J., Travnickova, M., Musilkova, J., Pajorova, J., Slepicka, P., … Molitor, M. (2018). Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnology Advances, 36(4), 1111–1126. https://doi.org/10.1016/j.biotechadv.2018.03.011
Kolios, G., & Moodley, Y. (2013). Introduction to Stem Cells and Regenerative Medicine. Respiration, 85(1), 3–10. https://doi.org/10.1159/000345615
Fan, Y., Chong, Y. S., Choolani, M. A., Cregan, M. D., & Chan, J. K. Y. (2010). Unravelling the Mystery of Stem/Progenitor Cells in Human Breast Milk. PLoS ONE, 5(12), e14421. https://doi.org/10.1371/journal.pone.0014421.
Hassiotou, F., Beltran, A., Chetwynd, E., Stuebe, A. M., Twigger, A.-J., Metzger, P., … Hartmann, P. E. (2012). Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells (Dayton, Ohio), 30(10), 2164–2174. https://doi.org/10.1002/stem.1188.
Hosseini, S. M., Talaei-khozani, T., Sani, M., & Owrangi, B. (2014). Differentiation of Human Breast-Milk Stem Cells to Neural Stem Cells and Neurons. Neurology Research International, 2014, 807896. https://doi.org/10.1155/2014/807896.
Sani, M., Hosseini, S. M., Salmannejad, M., Aleahmad, F., Ebrahimi, S., Jahanshahi, S., & Talaei-Khozani, T. (2015). Origins of the breast milk-derived cells; an endeavor to find the cell sources. Cell Biology International, 39(5), 611–618. https://doi.org/10.1002/cbin.10432
Tang, C., Zhou, Q., Lu, C., Xiong, M., & Lee, S. (2019). Comparison and culturing different types of cells from fresh breast milk with different culture medium. Pediatric Medicine, 2(0). https://doi.org/10.21037/pm.2019.02.02.
Li, S., Zhang, L., Zhou, Q., Jiang, S., Yang, Y., & Cao, Y. (2019). Characterization of Stem Cells and Immune Cells in Preterm and Term Mother’s Milk. Journal of Human Lactation, 35(3), 528–534. https://doi.org/10.1177/0890334419838986
Goudarzi, N., Shabani, R., Ebrahimi, M., Baghestani, A., Dehdashtian, E., Vahabzadeh, G., … Katebi, M. (2020). Comparative phenotypic characterization of human colostrum and breast milk-derived stem cells. Human Cell, 33(2), 308–317. https://doi.org/10.1007/s13577-019-00320-x.
Borhani-Haghighi, M., Navid, S., & Mohamadi, Y. (2020). The Therapeutic Potential of Conditioned Medium from Human Breast Milk Stem Cells in Treating Spinal Cord Injury. Asian Spine Journal, 14(2), 131–138. https://doi.org/10.31616/asj.2019.0026.
Gleeson, J. P., Chaudhary, N., Fein, K. C., Doerfler, R., Hredzak-Showalter, P., & Whitehead, K. A. (2022). Profiling of mature-stage human breast milk cells identifies six unique lactocyte subpopulations. Science Advances, 8(26), eabm6865. https://doi.org/10.1126/sciadv.abm6865.
Sani, M., Ebrahimi, S., Aleahmad, F., Salmannejad, M., Hosseini, S. M., Mazarei, G., & Talaei-Khozani, T. (2017). Differentiation potential of breast milk-derived mesenchymal stem cells into hepatocyte-like cells. Tissue Engineering and Regenerative Medicine, 14(5), 587–593. https://doi.org/10.1007/s13770-017-0066-x
Widjaja, S. L., Salimo, H., & Yulianto, I. (n.d.). Problems in Breastmilk Cell Isolation, 9.
Li, S., Zhang, L., Zhou, Q., Jiang, S., Yang, Y., & Cao, Y. (2019). Characterization of Stem Cells and Immune Cells in Preterm and Term Mother’s Milk. Journal of Human Lactation: Official Journal of International Lactation Consultant Association, 35(3), 528–534. https://doi.org/10.1177/0890334419838986
Acknowledgements
The authors would like to thank the Department of Science and Technology, "Innovation in Science Pursuit for Inspired Research (INSPIRE)" Government of India, for their support.
Author information
Authors and Affiliations
Contributions
Pooja Kumari: Conceptualization, Writing—Original Draft, Data Curation, Writing—Review & Editing; Aayushi Raval: Writing—Original Draft, Data collection, Reviewing and Editing; Pranav Rana: Reviewing and Editing; Sanjeev Kumar Mahto: Supervision, Project Administration, Reviewing and Editing.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The author declares no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, P., Raval, A., Rana, P. et al. Regenerative Potential of Human Breast Milk: A Natural Reservoir of Nutrients, Bioactive Components and Stem cells. Stem Cell Rev and Rep 19, 1307–1327 (2023). https://doi.org/10.1007/s12015-023-10534-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10534-0