Abstract
Natural killer (NK) cells are one of the innate immune cells that play an important role in preventing and controlling tumors and viral diseases, but their role in hematopoietic stem cell transplantation (HCT) is not yet fully understood. However, according to some research, these cells can prevent infections and tumor relapse without causing graft versus host disease (GVHD). In addition to NK cells, several studies are about the anti-leukemia effects of NK cell-derived exosomes that can highlight their roles in graft-versus-leukemia (GVL). In this paper, we intend to investigate the results of various articles on the role of NK cells in allogeneic hematopoietic cell transplantation and also their exosomes in GVL. Also, we have discussed the antiviral effects of these cells in post-HCT cytomegalovirus infection.
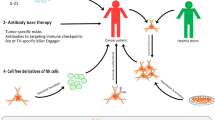
Graphical Abstract







Similar content being viewed by others
Data Availability
The data supporting the conclusions of this article are all online.
References
Herberman, R. B., Nunn, M. E., & Lavrin, D. H. (1975). Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. International Journal of Cancer, 16(2), 216–229.
Kiessling, R., Klein, E., & Wigzell, H. (1975). “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. European Journal of Immunology, 5(2), 112–117.
Miller, J. S., McCullar, V., Punzel, M., Lemischka, I. R., & Moore, K. A. (1999). Single adult human CD34+/Lin–/CD38 – progenitors give rise to natural killer cells, B-lineage cells, dendritic cells, and myeloid cells. Blood the Journal of the American Society of Hematology, 93(1), 96–106.
Luetke-Eversloh, M., Killig, M., & Romagnani, C. (2013). Signatures of human NK cell development and terminal differentiation. Frontiers in Immunology, 4, 499.
Vacca, P., Montaldo, E., Croxatto, D., Moretta, F., Bertaina, A., Vitale, C., et al. (2016). NK cells and other innate lymphoid cells in hematopoietic stem cell transplantation. Frontiers in Immunology, 7, 188.
Abrahamsen, I. W., Somme, S., Heldal, D., Egeland, T., Kvale, D., & Tjonnfjord, G. (2005). Immune reconstitution after allogeneic stem cell transplantation: the impact of stem cell source and graft-versus-host disease. Haematologica, 90(1), 86–93.
de Koning, C., Plantinga, M., Besseling, P., Boelens, J. J., & Nierkens, S. (2016). Immune reconstitution after allogeneic hematopoietic cell transplantation in children. Biology of Blood and Marrow Transplantation, 22(2), 195–206.
Lupo, K. B., & Matosevic, S. (2019). Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers, 11(6), 769.
Vitale, M., Cantoni, C., Della Chiesa, M., Ferlazzo, G., Carlomagno, S., Pende, D., et al. (2019). An historical overview: the discovery of how NK cells can kill enemies, recruit defense troops, and more. Frontiers in Immunology, 10, 1415.
Yu, Y., Kumar, V., & Bennett, M. (1992). Murine natural killer cells and marrow graft rejection. Annual Review of Immunology, 10(1), 189–213.
Taghavi-Farahabadi, M., Mahmoudi, M., Mahdaviani, S. A., Baghaei, K., Rayzan, E., Hashemi, S. M., et al. (2020). Improving the function of neutrophils from chronic granulomatous disease patients using mesenchymal stem cells’ exosomes. Human Immunology, 81(10–11), 614–624.
Hazrati, A., Soudi, S., Malekpour, K., Mahmoudi, M., Rahimi, A., Hashemi, S. M., et al. (2022). Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications. Biomarker Research, 10(1), 1–25.
Gill, S., Olson, J. A., & Negrin, R. S. (2009). Natural killer cells in allogeneic transplantation: effect on engraftment, graft-versus-tumor, and graft-versus-host responses. Biology of Blood and Marrow Transplantation, 15(7), 765–776.
Locatelli, F., Pende, D., Maccario, R., Mingari, M. C., Moretta, A., & Moretta, L. (2009). Haploidentical hemopoietic stem cell transplantation for the treatment of high-risk leukemias: how NK cells make the difference. Clinical Immunology, 133(2), 171–178.
Abel, A. M., Yang, C., Thakar, M. S., & Malarkannan, S. (2018). Natural killer cells: development, maturation, and clinical utilization. Frontiers in Immunology, 9, 1869.
Cooper, M. A., Fehniger, T. A., & Caligiuri, M. A. (2001). The biology of human natural killer-cell subsets. Trends in Immunology, 22(11), 633–640.
Ullah, M. A., Hill, G. R., & Tey, S. K. (2016). Functional reconstitution of natural killer cells in allogeneic hematopoietic stem cell transplantation. Frontiers in Immunology, 7, 144.
Fauriat, C., Long, E. O., Ljunggren, H. G., & Bryceson, Y. T. (2010). Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood, 115(11), 2167–2176.
Fehniger, T. A., Cooper, M. A., Nuovo, G. J., Cella, M., Facchetti, F., Colonna, M., et al. (2003). CD56bright natural killer cells are present in human lymph nodes and are activated by T cell–derived IL-2: a potential new link between adaptive and innate immunity. Blood the Journal of the American Society of Hematology, 101(8), 3052–3057.
Ferlazzo, G., Thomas, D., Lin, S. L., Goodman, K., Morandi, B., Muller, W. A., et al. (2004). The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. The Journal of Immunology, 172(3), 1455–1462.
Dogra, P., Rancan, C., Ma, W., Toth, M., Senda, T., Carpenter, D. J., et al. (2020). Tissue determinants of human NK cell development, function, and residence. Cell, 180(4), 749–763. e13.
Ali, T. H., Pisanti, S., Ciaglia, E., Mortarini, R., Anichini, A., Garofalo, C., et al. (2014). Enrichment of CD56 dim KIR + CD57 + highly cytotoxic NK cells in tumour-infiltrated lymph nodes of melanoma patients. Nature Communications, 5(1), 1–9.
Jacobs, R., Hintzen, G., Kemper, A., Beul, K., Kempf, S., Behrens, G., et al. (2001). CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. European Journal of Immunology, 31(10), 3121–3126.
Björkström, N. K., Riese, P., Heuts, F., Andersson, S., Fauriat, C., Ivarsson, M. A., et al. (2010). Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood, 116(19), 3853–3864.
Wu, F., Xie, M., Hun, M., She, Z., Li, C., Luo, S., et al. (2021). Natural killer cell-derived extracellular vesicles: novel players in cancer immunotherapy. Frontiers in Immunology, 12, 1970.
Wu, C. H., Li, J., Li, L., Sun, J., Fabbri, M., Wayne, A. S., et al. (2019). Extracellular vesicles derived from natural killer cells use multiple cytotoxic proteins and killing mechanisms to target cancer cells. Journal of Extracellular Vesicles, 8(1), 1588538.
Neviani, P., Wise, P. M., Murtadha, M., Liu, C. W., Wu, C. H., Jong, A. Y., et al. (2019). Natural killer–derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Research, 79(6), 1151–1164.
Lugini, L., Cecchetti, S., Huber, V., Luciani, F., Macchia, G., Spadaro, F., et al. (2012). Immune surveillance properties of human NK cell-derived exosomes. The Journal of Immunology, 189(6), 2833–2842.
Zhu, L., Kalimuthu, S., Oh, J. M., Gangadaran, P., Baek, S. H., Jeong, S. Y., et al. (2019). Enhancement of antitumor potency of extracellular vesicles derived from natural killer cells by IL-15 priming. Biomaterials, 190, 38–50.
Shilling, H. G., McQueen, K. L., Cheng, N. W., Shizuru, J. A., Negrin, R. S., & Parham, P. (2003). Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood the Journal of the American Society of Hematology, 101(9), 3730–3740.
Chang, Y. J., Zhao, X. Y., & Huang, X. J. (2008). Effects of the NK cell recovery on outcomes of unmanipulated haploidentical blood and marrow transplantation for patients with hematologic malignancies. Biology of Blood and Marrow Transplantation, 14(3), 323–334.
Suen, W. C. W., Lee, W. Y. W., Leung, K. T., Pan, X. H., & Li, G. (2018). Natural killer cell-based cancer immunotherapy: a review on 10 years completed clinical trials. Cancer Investigation, 36(8), 431–457.
Velardi, A., Ruggeri, L., Mancusi, A., Aversa, F., & Christiansen, F. T. (2009). Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Current Opinion in Immunology, 21(5), 525–530.
Parham, P. (2005). MHC class I molecules and KIRs in human history, health and survival. Nature Reviews Immunology, 5(3), 201–214.
Pyo, C. W., Wang, R., Vu, Q., Cereb, N., Yang, S. Y., Duh, F. M., et al. (2013). Recombinant structures expand and contract inter and intragenic diversification at the KIR locus. BMC Genomics, 14(1), 89.
Shilling, H. G., Young, N., Guethlein, L. A., Cheng, N. W., Gardiner, C. M., Tyan, D., et al. (2002). Genetic control of human NK cell repertoire. The Journal of Immunology, 169(1), 239–247.
Uhrberg, M., Valiante, N. M., Shum, B. P., Shilling, H. G., Lienert-Weidenbach, K., Corliss, B., et al. (1997). Human diversity in killer cell inhibitory receptor genes. Immunity, 7(6), 753–763.
Winter, C. C., Gumperz, J. E., Parham, P., Long, E. O., & Wagtmann, N. (1998). Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. The Journal of Immunology, 161(2), 571–577.
Moesta, A. K., & Parham, P. (2012). Diverse functionality among human NK cell receptors for the C1 epitope of HLA-C: KIR2DS2, KIR2DL2, and KIR2DL3. Frontiers in Immunology, 3, 336.
Rajagopalan, S., & Long, E. O. (2012). KIR2DL4 (CD158d): an activation receptor for HLA-G. Frontiers in Immunology, 3, 258.
Carr, W. H., Pando, M. J., & Parham, P. (2005). KIR3DL1 polymorphisms that affect NK cell inhibition by HLA-Bw4 ligand. The Journal of Immunology, 175(8), 5222–5229.
Goodridge, J. P., Burian, A., Lee, N., & Geraghty, D. E. (2013). HLA-F and MHC class I open conformers are ligands for NK cell Ig-like receptors. The Journal of Immunology, 191(7), 3553–3562.
Hansasuta, P., Dong, T., Thananchai, H., Weekes, M., Willberg, C., Aldemir, H., et al. (2004). Recognition of HLA-A3 and HLA‐A11 by KIR3DL2 is peptide‐specific. European Journal of Immunology, 34(6), 1673–1679.
Thielens, A., Vivier, E., & Romagné, F. (2012). NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Current Opinion in Immunology, 24(2), 239–245.
Moretta, A., Bottino, C., Pende, D., Tripodi, G., Tambussi, G., Viale, O., et al. (1990). Identification of four subsets of human CD3-CD16 + natural killer (NK) cells by the expression of clonally distributed functional surface molecules: correlation between subset assignment of NK clones and ability to mediate specific alloantigen recognition. The Journal of Experimental Medicine, 172(6), 1589–1598.
Ljunggren, H. G., & Kärre, K. (1990). In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunology Today, 11, 237–244.
Davies, S. M., Ruggieri, L., DeFor, T., Wagner, J. E., Weisdorf, D. J., Miller, J. S., et al. (2002). Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Blood, 100(10), 3825–3827.
Symons, H. J., Leffell, M. S., Rossiter, N. D., Zahurak, M., Jones, R. J., & Fuchs, E. J. (2010). Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biology of Blood and Marrow Transplantation, 16(4), 533–542.
Pende, D., Marcenaro, S., Falco, M., Martini, S., Bernardo, M. E., Montagna, D., et al. (2009). Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood the Journal of the American Society of Hematology, 113(13), 3119–3129.
Clausen, J., Wolf, D., Petzer, A., Gunsilius, E., Schumacher, P., Kircher, B., et al. (2007). Impact of natural killer cell dose and donor killer-cell immunoglobulin‐like receptor (KIR) genotype on outcome following human leucocyte antigen‐identical haematopoietic stem cell transplantation. Clinical & Experimental Immunology, 148(3), 520–528.
Wang, W., Erbe, A. K., DeSantes, K. B., & Sondel, P. M. (2017). Donor selection for ex vivo-expanded natural killer cells as adoptive cancer immunotherapy. Future Oncology, 13(12), 1043–1047. https://doi.org/10.2217/fon-2017-0039
Sivori, S., Falco, M., Carlomagno, S., Romeo, E., Soldani, C., Bensussan, A., et al. (2010). A novel KIR-associated function: evidence that CpG DNA uptake and shuttling to early endosomes is mediated by KIR3DL2. Blood, 116(10), 1637–1647.
Jiang, W., Johnson, C., Jayaraman, J., Simecek, N., Noble, J., Moffatt, M. F., et al. (2012). Copy number variation leads to considerable diversity for B but not A haplotypes of the human KIR genes encoding NK cell receptors. Genome Research, 22(10), 1845–1854.
Martin, A. M., Kulski, J. K., Gaudieri, S., Witt, C. S., Freitas, E. M., Trowsdale, J., et al. (2004). Comparative genomic analysis, diversity and evolution of two KIR haplotypes A and B. Gene, 335, 121–131.
Bachanova, V., Weisdorf, D. J., Wang, T., Marsh, S. G., Trachtenberg, E., Haagenson, M. D., et al. (2016). Donor KIR B genotype improves progression-free survival of non-Hodgkin lymphoma patients receiving unrelated donor transplantation. Biology of Blood and Marrow Transplantation, 22(9), 1602–1607.
Impola, U., Turpeinen, H., Alakulppi, N., Linjama, T., Volin, L., Niittyvuopio, R., et al. (2014). Donor haplotype B of NK KIR receptor reduces the relapse risk in HLA-identical sibling hematopoietic stem cell transplantation of AML patients. Frontiers in Immunology, 5, 405.
Oevermann, L., Michaelis, S. U., Mezger, M., Lang, P., Toporski, J., Bertaina, A., et al. (2014). KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood the Journal of the American Society of Hematology, 124(17), 2744–2747.
Cooley, S., Weisdorf, D. J., Guethlein, L. A., Klein, J. P., Wang, T., Le, C. T., et al. (2010). Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood, 116(14), 2411–2419.
Pyo, C. W., Guethlein, L. A., Vu, Q., Wang, R., Abi-Rached, L., Norman, P. J., et al. (2010). Different patterns of evolution in the centromeric and telomeric regions of group A and B haplotypes of the human killer cell Ig-like receptor locus. PLoS One, 5(12), e15115.
Ryan, J. C., & Seaman, W. E. (1997). Divergent functions of lectin-like receptors on NK cells. Immunological Reviews, 155(1), 79–89.
Braud, V. M., Allan, D. S., O’Callaghan, C. A., Söderström, K., D’Andrea, A., Ogg, G. S., et al. (1998). HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature, 391(6669), 795–799.
Lee, N., Llano, M., Carretero, M., Ishitani, A., Navarro, F., López-Botet, M., et al. (1998). HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proceedings of the National Academy of Sciences, 95(9), 5199–204.
Freud, A. G., Yokohama, A., Becknell, B., Lee, M. T., Mao, H. C., Ferketich, A. K., et al. (2006). Evidence for discrete stages of human natural killer cell differentiation in vivo. The Journal of Experimental Medicine, 203(4), 1033–1043.
Nguyen, S., Dhedin, N., Vernant, J. P., Kuentz, M., Jijakli, A. A., Rouas-Freiss, N., et al. (2005). NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood, 105(10), 4135–4142.
Ruggeri, L., Urbani, E., André, P., Mancusi, A., Tosti, A., Topini, F., et al. (2016). Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica, 101(5), 626–633.
López-Botet, M., Muntasell, A., & Vilches, C. (Eds.). (2014). The CD94/NKG2C + NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection. Seminars in Immunology. Elsevier.
Bauer, S., Groh, V., Wu, J., Steinle, A., Phillips, J. H., Lanier, L. L., et al. (1999). Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science, 285(5428), 727–729.
Billadeau, D. D., Upshaw, J. L., Schoon, R. A., Dick, C. J., & Leibson, P. J. (2003). NKG2D-DAP10 triggers human NK cell–mediated killing via a Syk-independent regulatory pathway. Nature Immunology, 4(6), 557–564.
Garrity, D., Call, M. E., Feng, J., & Wucherpfennig, K. W. (2005). The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proceedings of the National Academy of Sciences, 102(21), 7641–6.
Bottino, C., Biassoni, R., Millo, R., Moretta, L., & Moretta, A. (2000). The human natural cytotoxicity receptors (NCR) that induce HLA class I-independent NK cell triggering. Human Immunology, 61(1), 1–6.
Bottino, C., Moretta, L., & Moretta, A. (2006). NK cell activating receptors and tumor recognition in humans. Immunobiology of Natural Killer Cell Receptors (p. 175–82). Springer.
Cantoni, C., Bottino, C., Vitale, M., Pessino, A., Augugliaro, R., Malaspina, A., et al. (1999). NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. The Journal of Experimental Medicine, 189(5), 787–796.
Pende, D., Parolini, S., Pessino, A., Sivori, S., Augugliaro, R., Morelli, L., et al. (1999). Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. The Journal of Experimental Medicine, 190(10), 1505–1516.
Sivori, S., Pende, D., Bottino, C., Marcenaro, E., Pessino, A., Biassoni, R., et al. (1999). NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. European Journal of Immunology, 29(5), 1656–1666.
Barrow, A. D., Edeling, M. A., Trifonov, V., Luo, J., Goyal, P., Bohl, B., et al. (2018). Natural killer cells control tumor growth by sensing a growth factor. Cell, 172(3), 534–548. e19.
Baychelier, F., Sennepin, A., Ermonval, M., Dorgham, K., Debré, P., & Vieillard, V. (2013). Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood. The Journal of the American Society of Hematology, 122(17), 2935–2942.
Brandt, C. S., Baratin, M., Yi, E. C., Kennedy, J., Gao, Z., Fox, B., et al. (2009). The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. Journal of Experimental Medicine, 206(7), 1495–1503.
Rosental, B., Brusilovsky, M., Hadad, U., Oz, D., Appel, M. Y., Afergan, F., et al. (2011). Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. The Journal of Immunology, 187(11), 5693–5702.
von Strandmann, E. P., Simhadri, V. R., von Tresckow, B., Sasse, S., Reiners, K. S., Hansen, H. P., et al. (2007). Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity, 27(6), 965–974.
Narni-Mancinelli, E., Gauthier, L., Baratin, M., Guia, S., Fenis, A., Deghmane, A. E., Rossi, B., Fourquet, P., Escalière, B., Kerdiles, Y. M., Ugolini, S., Taha, M. K., & Vivier, E. (2017). Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Science Immunology, 2(10), eaam9628. https://doi.org/10.1126/sciimmunol.aam9628
Del Zotto, G., Marcenaro, E., Vacca, P., Sivori, S., Pende, D., Della Chiesa, M., et al. (2017). Markers and function of human NK cells in normal and pathological conditions. Cytometry Part B: Clinical Cytometry, 92(2), 100–114.
Fauriat, C., Just-Landi, S., Mallet, F., Arnoulet, C., Sainty, D., Olive, D., et al. (2007). Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood, 109(1), 323–330.
Triplett, B. M., Horwitz, E. M., Iyengar, R., Turner, V., Holladay, M. S., Gan, K., et al. (2009). Effects of activating NK cell receptor expression and NK cell reconstitution on the outcomes of unrelated donor hematopoietic cell transplantation for hematologic malignancies. Leukemia, 23(7), 1278–1287.
Di Pace, A. L., Tumino, N., Besi, F., Alicata, C., Conti, L. A., Munari, E., et al. (2020). Characterization of human NK cell-derived exosomes: Role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers, 12(3), 661.
Bottino, C., Castriconi, R., Pende, D., Rivera, P., Nanni, M., Carnemolla, B., et al. (2003). Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. The Journal of Experimental Medicine, 198(4), 557–567.
Martinet, L., & Smyth, M. J. (2015). Balancing natural killer cell activation through paired receptors. Nature Reviews Immunology, 15(4), 243–254.
Zhang, Q., Bi, J., Zheng, X., Chen, Y., Wang, H., Wu, W., et al. (2018). Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nature Immunology, 19(7), 723–732.
Bowles, J. A., Wang, S. Y., Link, B. K., Allan, B., Beuerlein, G., Campbell, M. A., et al. (2006). Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood, 108(8), 2648–2654.
Vivier, E., Raulet, D. H., Moretta, A., Caligiuri, M. A., Zitvogel, L., Lanier, L. L., et al. (2011). Innate or adaptive immunity? The example of natural killer cells. Science, 331(6013), 44–49.
Colonna, M., Navarro, F., Bellón, T., Llano, M., García, P., Samaridis, J., et al. (1997). A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. The Journal of Experimental Medicine, 186(11), 1809–1818.
Bessoles, S., Grandclément, C., Alari-Pahissa, E., Gehrig, J., Jeevan-Raj, B., & Held, W. (2014). Adaptations of natural killer cells to self-MHC class I. Frontiers in Immunology, 5, 349.
Stern, M., Passweg, J., Meyer-Monard, S., Esser, R., Tonn, T., Soerensen, J., et al. (2013). Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplantation, 48(3), 433–438.
Juelke, K., Killig, M., Thiel, A., Dong, J., & Romagnani, C. (2009). Education of hyporesponsive NK cells by cytokines. European Journal of Immunology, 39(9), 2548–2555.
Shoae-Hassani, A., Hamidieh, A. A., Behfar, M., Mohseni, R., Mortazavi-Tabatabaei, S. A., & Asgharzadeh, S. (2017). NK cell-derived exosomes from NK cells previously exposed to neuroblastoma cells augment the antitumor activity of cytokine-activated NK cells. Journal of Immunotherapy, 40(7), 265–276. https://doi.org/10.1097/CJI.0000000000000179
Jong, A. Y., Wu, C. H., Li, J., Sun, J., Fabbri, M., Wayne, A. S., et al. (2017). Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. Journal of Extracellular Vesicles, 6(1), 1294368.
Choi, J. W., Lim, S., Kang, J. H., Hwang, S. H., Hwang, K. C., Kim, S. W., et al. (2020). Proteome analysis of human natural killer cell derived extracellular vesicles for identification of anticancer effectors. Molecules, 25(21), 5216.
Elliott, J. M., & Yokoyama, W. M. (2011). Unifying concepts of MHC-dependent natural killer cell education. Trends in Immunology, 32(8), 364–372.
Raulet, D. H., & Vance, R. E. (2006). Self-tolerance of natural killer cells. Nature Reviews Immunology, 6(7), 520–531.
Shifrin, N., Raulet, D. H., & Ardolino, M. (Eds.). (2014). NK cell self tolerance, responsiveness and missing self recognition. Seminars in immunology. Elsevier.
Raulet, D. H. (Ed.). (2006). editor Missing self recognition and self tolerance of natural killer (NK) cells. Seminars in Immunology. Elsevier.
Jonsson, A. H., & Yokoyama, W. M. (2009). Natural killer cell tolerance: licensing and other mechanisms. Advances in Immunology, 101, 27–79.
Joncker, N. T., Fernandez, N. C., Treiner, E., Vivier, E., & Raulet, D. H. (2009). NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. The Journal of Immunology, 182(8), 4572–4580.
Foley, B., Cooley, S., Verneris, M. R., Curtsinger, J., Luo, X., Waller, E. K., et al. (2011). NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood the Journal of the American Society of Hematology, 118(10), 2784–2792.
Yu, J., Venstrom, J. M., Liu, X. R., Pring, J., Hasan, R. S., O’Reilly, R. J., et al. (2009). Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell–depleted allogeneic hematopoietic cell transplantation. Blood, 113(16), 3875–3884.
Orr, M. T., Murphy, W. J., & Lanier, L. L. (2010). ’Unlicensed’natural killer cells dominate the response to cytomegalovirus infection. Nature Immunology, 11(4), 321–327.
Zamora, A. E., Aguilar, E. G., Sungur, C. M., Khuat, L. T., Dunai, C., Lochhead, G. R., Du, J., Pomeroy, C., Blazar, B. R., Longo, D. L., Venstrom, J. M., Baumgarth, N., & Murphy, W. J. (2017). Licensing delineates helper and effector NK cell subsets during viral infection. JCI Insight, 2(10), e87032. https://doi.org/10.1172/jci.insight.87032
Yokoyama, W. M., & Kim, S. (2006). Licensing of natural killer cells by self-major histocompatibility complex class I. Immunological Reviews, 214(1), 143–154.
Björklund, A. T., Schaffer, M., Fauriat, C., Ringdén, O., Remberger, M., Hammarstedt, C., et al. (2010). NK cells expressing inhibitory KIR for non–self-ligands remain tolerant in HLA-matched sibling stem cell transplantation. Blood the Journal of the American Society of Hematology, 115(13), 2686–2694.
He, Y., & Tian, Z. (2017). NK cell education via nonclassical MHC and non-MHC ligands. Cellular & Molecular Immunology, 14(4), 321–330.
Tripathy, S. K., Keyel, P. A., Yang, L., Pingel, J. T., Cheng, T. P., Schneeberger, A., et al. (2008). Continuous engagement of a self-specific activation receptor induces NK cell tolerance. The Journal of Experimental Medicine, 205(8), 1829–1841.
Chewning, J. H., Gudme, C. N., Hsu, K. C., Selvakumar, A., & Dupont, B. (2007). KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. The Journal of Immunology, 179(2), 854–868.
Venstrom, J. M., Pittari, G., Gooley, T. A., Chewning, J. H., Spellman, S., Haagenson, M., et al. (2012). HLA-C–dependent prevention of leukemia relapse by donor activating KIR2DS1. New England Journal of Medicine, 367(9), 805–816.
Alvarez, M., Sun, K., & Murphy, W. J. (2016). Mouse host unlicensed NK cells promote donor allogeneic bone marrow engraftment. Blood, 127(9), 1202–1205.
Olson, J. A., Leveson-Gower, D. B., Gill, S., Baker, J., Beilhack, A., & Negrin, R. S. (2010). NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood, 115(21), 4293–4301.
Ruggeri, L., Capanni, M., Urbani, E., Perruccio, K., Shlomchik, W. D., Tosti, A., et al. (2002). Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science, 295(5562), 2097–2100.
Simonetta, F., Alvarez, M., & Negrin, R. S. (2017). Natural killer cells in graft-versus-host-disease after allogeneic hematopoietic cell transplantation. Frontiers in Immunology, 8, 465.
Bunting, M. D., Varelias, A., Souza-Fonseca-Guimaraes, F., Schuster, I. S., Lineburg, K. E., Kuns, R. D., et al. (2017). GVHD prevents NK-cell–dependent leukemia and virus-specific innate immunity. Blood the Journal of the American Society of Hematology, 129(5), 630–642.
Nguyen, S., Kuentz, M., Vernant, J., Dhedin, N., Bories, D., Debre, P., et al. (2008). Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem-cell transplantation. Leukemia, 22(2), 344–352.
Caligiuri, M. A. (2008). Human natural killer cells. Blood. The Journal of the American Society of Hematology, 112(3), 461–469.
Marcenaro, E., Cantoni, C., Pesce, S., Prato, C., Pende, D., Agaugué, S., et al. (2009). Uptake of CCR7 and acquisition of migratory properties by human KIR + NK cells interacting with monocyte-derived DC or EBV cell lines: regulation by KIR/HLA-class I interaction. Blood the Journal of the American Society of Hematology, 114(19), 4108–4116.
Trivedi, P. P., Roberts, P. C., Wolf, N. A., & Swanborg, R. H. (2005). NK cells inhibit T cell proliferation via p21-mediated cell cycle arrest. The Journal of Immunology, 174(8), 4590–4597.
Cerboni, C., Zingoni, A., Cippitelli, M., Piccoli, M., Frati, L., & Santoni, A. (2007). Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK-cell lysis. Blood, 110(2), 606–615.
Rabinovich, B. A., Li, J., Shannon, J., Hurren, R., Chalupny, J., Cosman, D., et al. (2003). Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. The Journal of Immunology, 170(7), 3572–3576.
Ghadially, H., Ohana, M., Elboim, M., Gazit, R., Gur, C., Nagler, A., et al. (2014). NK cell receptor NKp46 regulates graft-versus-host disease. Cell Reports, 7(6), 1809–1814.
Venstrom, J. M., Gooley, T. A., Spellman, S., Pring, J., Malkki, M., Dupont, B., et al. (2010). Donor activating KIR3DS1 is associated with decreased acute GVHD in unrelated allogeneic hematopoietic stem cell transplantation. Blood the Journal of the American Society of Hematology, 115(15), 3162–3165.
Huntington, N. D. (2014). The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunology and Cell Biology, 92(3), 210–213.
Meguro, A., Ozaki, K., Oh, I., Hatanaka, K., Matsu, H., Tatara, R., et al. (2010). IL-21 is critical for GVHD in a mouse model. Bone Marrow Transplantation, 45(4), 723–729.
Pratt, L., Liu, Y., Ugarte-Torres, A., Hoegh-Petersen, M., Podgorny, P., Lyon, A., et al. (2013). IL15 levels on day 7 after hematopoietic cell transplantation predict chronic GVHD. Bone Marrow Transplantation, 48(5), 722–728.
Chan, Y. L. T., Zuo, J., Inman, C., Croft, W., Begum, J., Croudace, J., et al. (2018). NK cells produce high levels of IL-10 early after allogeneic stem cell transplantation and suppress development of acute GVHD. European Journal of Immunology, 48(2), 316–329.
Hu, L. J., Zhao, X. Y., Yu, X. X., Lv, M., Han, T. T., Han, W., et al. (2019). Quantity and quality reconstitution of NKG2A + natural killer cells are associated with graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation, 25(1), 1–11.
Bao, X., Hou, L., Sun, A., Qiu, Q., Yuan, X., Chen, M., et al. (2010). The impact of KIR2DS4 alleles and the expression of KIR in the development of acute GVHD after unrelated allogeneic hematopoietic SCT. Bone Marrow Transplantation, 45(9), 1435–1441.
Hosokai, R., Masuko, M., Shibasaki, Y., Saitoh, A., Furukawa, T., & Imai, C. (2017). Donor killer immunoglobulin-like receptor haplotype B/x induces severe acute graft-versus-host disease in the presence of human leukocyte antigen mismatch in T cell–replete hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation, 23(4), 606–611.
Nishimura, R., Baker, J., Beilhack, A., Zeiser, R., Olson, J. A., Sega, E. I., et al. (2008). In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood the Journal of the American Society of Hematology, 112(6), 2563–2574.
Olson, J. A., Zeiser, R., Beilhack, A., Goldman, J. J., & Negrin, R. S. (2009). Tissue-specific homing and expansion of donor NK cells in allogeneic bone marrow transplantation. The Journal of Immunology, 183(5), 3219–3228.
Zhang, P., Yang, S., Zou, Y., Yan, X., Wu, H., Zhou, M., et al. (2019). NK cell predicts the severity of acute graft-versus-host disease in patients after allogeneic stem cell transplantation using antithymocyte globulin (ATG) in pretreatment scheme. BMC Immunology, 20(1), 46.
Mehta, R. S., Saliba, R. M., Chen, J., Rondon, G., Hammerstrom, A. E., Alousi, A., et al. (2016). Post-transplantation cyclophosphamide versus conventional graft‐versus‐host disease prophylaxis in mismatched unrelated donor haematopoietic cell transplantation. British Journal of Haematology, 173(3), 444–455.
Scholl, S., Mügge, L., Issa, M. C., Kasper, C., Pachmann, K., Höffken, K., et al. (2005). Impact of early NK cell recovery on development of GvHD and CMV reactivation in dose-reduced regimen prior to allogeneic PBSCT. Bone Marrow Transplantation, 35(2), 183–190.
Huang, X., Zhao, X., Liu, D., Liu, K., & Xu, L. (2007). Deleterious effects of KIR ligand incompatibility on clinical outcomes in haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion. Leukemia, 21(4), 848–851.
Lundqvist, A., Philip McCoy, J., Samsel, L., & Childs, R. (2007). Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49-mismatched NK cells from MHC-matched donors. Blood, 109(8), 3603–3606.
Asai, O., Longo, D. L., Tian, Z. G., Hornung, R. L., Taub, D. D., Ruscetti, F. W., et al. (1998). Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. The Journal of Clinical Investigation, 101(9), 1835–1842.
Yang, Y. G., Dey, B. R., Sergio, J. J., Pearson, D. A., & Sykes, M. (1998). Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. The Journal of Clinical Investigation, 102(12), 2126–2135.
Sweeney, C., & Vyas, P. (2019). The graft-versus-leukemia effect in AML. Frontiers in Oncology, 9, 1217.
Lanier, L. L. (2005). NK cell recognition. Annual Review of Immunology, 23, 225–274.
Aversa, F., Tabilio, A., Terenzi, A., Velardi, A., Falzetti, F., Giannoni, C., Iacucci, R., Zei, T., Martelli, M. P., & Gambelunghe, C. (1994). Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood, 84(11), 3948–3955.
Locatelli, F., Pende, D., Falco, M., Della Chiesa, M., Moretta, A., & Moretta, L. (2018). NK cells mediate a crucial graft-versus-leukemia effect in haploidentical-HSCT to cure high-risk acute leukemia. Trends in Immunology, 39(7), 577–590.
Arvindam, U. S., Aguilar, E. G., Felices, M., Murphy, W., & Miller, J. (2019). Natural Killer Cells in GvHD and GvL. Immune Biology of Allogeneic Hematopoietic Stem Cell Transplantation (pp. 275–292). Elsevier.
Retiere, C., Willem, C., Legrand, N., Guillaume, T., Gagne, K., Peterlin, P., et al. (2017). NK-cell alloreactivity is associated with acute Gvhd and decreased relapse incidence after T-replete haplo-identical allotransplant with high-dose post-transplant cyclophosphamide. Blood, 130(Supplement 1), 3262.
Willem, C., Makanga, D. R., Guillaume, T., Maniangou, B., Legrand, N., Gagne, K., et al. (2019). Impact of KIR/HLA incompatibilities on NK cell reconstitution and clinical outcome after T Cell–replete haploidentical hematopoietic stem cell transplantation with posttransplant cyclophosphamide. The Journal of Immunology, 202(7), 2141–2152.
Ruggeri, L., Mancusi, A., Capanni, M., Urbani, E., Carotti, A., Aloisi, T., et al. (2007). Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood, 110(1), 433–440.
Luznik, L., O’Donnell, P. V., & Fuchs, E. J. (Eds.). (2012). Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Seminars in oncology. Elsevier.
Shimoni, A., Labopin, M., Lorentino, F., Van Lint, M. T., Koc, Y., Gülbas, Z., et al. (2019). Killer cell immunoglobulin-like receptor ligand mismatching and outcome after haploidentical transplantation with post-transplant cyclophosphamide. Leukemia, 33(1), 230–239.
Hsu, K. C., Gooley, T., Malkki, M., Pinto-Agnello, C., Dupont, B., Bignon, J. D., et al. (2006). KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biology of Blood and Marrow Transplantation, 12(8), 828–836.
Hsu, K. C., Keever-Taylor, C. A., Wilton, A., Pinto, C., Heller, G., Arkun, K., et al. (2005). Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood, 105(12), 4878–4884.
Shaffer, B. C., & Hsu, K. C. (2016). How important is NK alloreactivity and KIR in allogeneic transplantation? Best Practice & Research Clinical Haematology, 29(4), 351–358.
Faridi, R. M., Kemp, T. J., Dharmani-Khan, P., Lewis, V., Tripathi, G., Rajalingam, R., et al. (2016). Donor-recipient matching for KIR genotypes reduces chronic GVHD and missing inhibitory KIR ligands protect against relapse after myeloablative, HLA matched hematopoietic cell transplantation. PLoS One, 11(6), e0158242.
Russo, A., Oliveira, G., Berglund, S., Greco, R., Gambacorta, V., Cieri, N., et al. (2018). NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: dynamics and clinical implications. Blood the Journal of the American Society of Hematology, 131(2), 247–262.
Wolschke, C., Stübig, T., Hegenbart, U., Schönland, S., Heinzelmann, M., Hildebrandt, Y., et al. (2013). Postallograft lenalidomide induces strong NK cell–mediated antimyeloma activity and risk for T cell–mediated GvHD: Results from a phase I/II dose-finding study. Experimental Hematology, 41(2), 134–142. e3.
Kheav, V. D., Busson, M., Scieux, C., de Latour, R. P., Maki, G., Haas, P., et al. (2014). Favorable impact of natural killer cell reconstitution on chronic graft-versus-host disease and cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Haematologica, 99(12), 1860–1867.
Liu, L. L., Béziat, V., Oei, V. Y., Pfefferle, A., Schaffer, M., Lehmann, S., et al. (2017). Ex vivo expanded adaptive NK cells effectively kill primary acute lymphoblastic leukemia cells. Cancer Immunology Research, 5(8), 654–665.
Sánchez-Martínez, D., Lanuza, P. M., Gómez, N., Muntasell, A., Cisneros, E., Moraru, M., et al. (2016). Activated allogeneic NK cells preferentially kill poor prognosis B-cell chronic lymphocytic leukemia cells. Frontiers in Immunology, 7, 454.
Torelli, G. F., Peragine, N., Raponi, S., Pagliara, D., De Propris, M. S., Vitale, A., et al. (2014). Recognition of adult and pediatric acute lymphoblastic leukemia blasts by natural killer cells. Haematologica, 99(7), 1248–1254.
Ureshino, H., Shindo, T., Sano, H., Kubota, Y., Ando, T., Kidoguchi, K., Kusaba, K., Itamura, H., Kojima, H., Kusunoki, Y., Miyazaki, Y., Kojima, K., Tanaka, H., Saji, H., Oshima, K., & Kimura, S. (2020). Reconstitution of NK cells expressing KIR3DL1 is associated with reduced NK cell activity and relapse of CML after allogeneic hematopoietic stem cell transplantation. International Journal of Hematology, 111(5), 733–738. https://doi.org/10.1007/s12185-019-02809-5
Mancusi, A., Ruggeri, L., Urbani, E., Pierini, A., Massei, M. S., Carotti, A., et al. (2015). Haploidentical hematopoietic transplantation from KIR ligand–mismatched donors with activating KIRs reduces nonrelapse mortality. Blood, 125(20), 3173–3182.
Marcenaro, E., Carlomagno, S., Pesce, S., Della Chiesa, M., Moretta, A., & Sivori, S. (2011). Role of alloreactive KIR2DS1 + NK cells in haploidentical hematopoietic stem cell transplantation. Journal of Leukocyte Biology, 90(4), 661–667.
Hu, B., He, Y., Wu, Y., Bao, G., Liu, H., Welniak, L. A., et al. (2010). Activated allogeneic NK cells as suppressors of alloreactive responses. Biology of Blood and Marrow Transplantation, 16(6), 772–781.
Wang, H., & Yang, Y. G. (2014). The complex and central role of interferon-γ in graft‐versus‐host disease and graft‐versus‐tumor activity. Immunological Reviews, 258(1), 30–44.
Yang, Y. G., Qi, J., Wang, M. G., & Sykes, M. (2002). Donor-derived interferon γ separates graft-versus-leukemia effects and graft-versus-host disease induced by donor CD8 T cells. Blood the Journal of the American Society of Hematology, 99(11), 4207–4215.
Gill, S., Vasey, A. E., De Souza, A., Baker, J., Smith, A. T., Kohrt, H. E., et al. (2012). Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood, 119(24), 5758–5768.
Simonetta, F., Pradier, A., Bosshard, C., Masouridi-Levrat, S., Chalandon, Y., & Roosnek, E. (2015). NK cell functional impairment after allogeneic hematopoietic stem cell transplantation is associated with reduced levels of T-bet and eomesodermin. The Journal of Immunology, 195(10), 4712–4720.
Wagner, J. A., Wong, P., Schappe, T., Berrien-Elliott, M. M., Cubitt, C., Jaeger, N., et al. (2020). Stage-Specific Requirement for Eomes in Mature NK Cell Homeostasis and Cytotoxicity. Cell Reports, 31(9), 107720.
Ghasemzadeh, M., Hosseini, E., Schwarer, A. P., & Pourfathollah, A. A. (2016). NK cell maturation to CD56dim subset associated with high levels of NCRs overrides the inhibitory effect of NKG2A and recovers impaired NK cell cytolytic potential after allogeneic hematopoietic stem cell transplantation. Leukemia Research, 43, 58–65.
Grzywacz, B., Kataria, N., Sikora, M., Oostendorp, R. A., Dzierzak, E. A., Blazar, B. R., et al. (2006). Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood, 108(12), 3824–3833.
Minculescu, L., Marquart, H. V., Friis, L. S., Petersen, S. L., Schiødt, I., Ryder, L. P., Andersen, N. S., & Sengeloev, H. (2016). Early natural killer cell reconstitution predicts overall survival in T cell-replete allogeneic hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation : Journal of the American Society for Blood and Marrow Transplantation, 22(12), 2187–2193. https://doi.org/10.1016/j.bbmt.2016.09.006
Minculescu, L., Fischer-Nielsen, A., Haastrup, E., Ryder, L. P., Andersen, N. S., Schjoedt, I., et al. (2020). Improved relapse-free survival in patients with high natural killer cell doses in grafts and during early immune reconstitution after allogeneic stem cell transplantation. Frontiers in Immunology, 11, 1068.
Hu, L., Zhao, X. Y., Yu, X., Lv, M., Han, T. T., Han, W., et al. (2018). Quantity and quality reconstitution of NKG2A + NK cells are associated with graft-versus-host disease after allogeneic hematopoietic cell transplantation. Blood, 132(Supplement 1), 4582.
Lang, P., Feuchtinger, T., Teltschik, H., Schwinger, W., Schlegel, P., Pfeiffer, M., et al. (2015). Improved immune recovery after transplantation of TCRαβ/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transplantation, 50(2), S6–S10.
Locatelli, F., Merli, P., Pagliara, D., Li Pira, G., Falco, M., Pende, D., et al. (2017). Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood, 130(5), 677–685.
Merli, P., Vacca, P., Galaverna, F., Tumino, N., Moretta, L., & Locatelli, F. (2020). TCRαβ/CD19 depleted hematopoietic stem cell transplantation from haploidentical donors: Dissecting the GvL/GvHD conundrum. Bone Marrow Transplantation, 55(7), 1483–1484. https://doi.org/10.1038/s41409-020-0891-8
Ciurea, S. O., Schafer, J. R., Bassett, R., Denman, C. J., Cao, K., Willis, D., et al. (2017). Phase 1 clinical trial using mbIL21 ex vivo–expanded donor-derived NK cells after haploidentical transplantation. Blood, 130(16), 1857–1868.
Leong, J. W., Chase, J. M., Romee, R., Schneider, S. E., Sullivan, R. P., Cooper, M. A., et al. (2014). Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biology of Blood and Marrow Transplantation, 20(4), 463–473.
Romee, R., Cooley, S., Berrien-Elliott, M. M., Westervelt, P., Verneris, M. R., Wagner, J. E., et al. (2018). First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood, 131(23), 2515–2527.
Song, Y., Hu, B., Liu, Y., Jin, Z., Zhang, Y., Lin, D., et al. (2018). IL-12/IL‐18‐preactivated donor NK cells enhance GVL effects and mitigate GvHD after allogeneic hematopoietic stem cell transplantation. European Journal of Immunology, 48(4), 670–682.
Davids, M. S., Kim, H. T., Bachireddy, P., Costello, C., Liguori, R., Savell, A., et al. (2016). Ipilimumab for patients with relapse after allogeneic transplantation. New England Journal Of Medicine, 375, 143–153.
Hattori, N., & Nakamaki, T. (2019). Natural killer immunotherapy for minimal residual disease eradication following allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. International Journal of Molecular Sciences, 20(9), 2057.
Hsu, J., Hodgins, J. J., Marathe, M., Nicolai, C. J., Bourgeois-Daigneault, M. C., Trevino, T. N., et al. (2018). Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. The Journal of Clinical Investigation, 128(10), 4654–4668.
Korde, N., Carlsten, M., Lee, M. J., Minter, A., Tan, E., Kwok, M., et al. (2014). A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica, 99(6), e81.
Sivori, S., Vacca, P., Del Zotto, G., Munari, E., Mingari, M. C., & Moretta, L. (2019). Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cellular & Molecular Immunology, 16(5), 430–441.
Gleason, M. K., Verneris, M. R., Todhunter, D. A., Zhang, B., McCullar, V., Zhou, S. X., et al. (2012). Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Molecular Cancer Therapeutics, 11(12), 2674–2684.
Gonzalez-Rodriguez, A. P., Villa-Álvarez, M., Sordo-Bahamonde, C., Lorenzo-Herrero, S., & Gonzalez, S. (2019). NK cells in the treatment of hematological malignancies. Journal of Clinical Medicine, 8(10), 1557.
Rader, C. (2020). Bispecific antibodies in cancer immunotherapy. Current Opinion in Biotechnology, 65, 9–16.
Tanaka, J., & Miller, J. S. (2020). Recent progress in and challenges in cellular therapy using NK cells for hematological malignancies. Blood Reviews, 44, 100678. https://doi.org/10.1016/j.blre.2020.100678
Lee, D. A., Denman, C. J., Rondon, G., Woodworth, G., Chen, J., Fisher, T., et al. (2016). Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biology of Blood and Marrow Transplantation, 22(7), 1290–1298.
Hu, Y., Tian, Z., & Zhang, C. (2018). Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacologica Sinica, 39(2), 167–176.
Vacca, P., Pietra, G., Tumino, N., Munari, E., Mingari, M. C., & Moretta, L. (2020). Exploiting human NK cells in tumor therapy. Frontiers in Immunology, 10, 3013.
Boyiadzis, M., Hong, C. S., & Whiteside, T. L. (2019). Anti-leukemia effects of NK cell-derived exosomes. Blood, 134, 3223.
Samara, A., Granot, G., Anbar, M., Raanani, P., & Rozovski, U. (2021). Using NK-derived exosomes to treat leukemia. Blood, 138, 1872.
Lieberman, J. (2010). Granzyme A activates another way to die. Immunological Reviews, 235(1), 93–104.
MacDonald, G., Shi, L., Velde, C. V., Lieberman, J., & Greenberg, A. H. (1999). Mitochondria-dependent and-independent regulation of granzyme B–induced apoptosis. The Journal of Experimental Medicine, 189(1), 131–144.
Sun, H., Shi, K., Qi, K., Kong, H., Zhang, J., Dai, S., Ye, W., Deng, T., He, Q., & Zhou, M. (2019). Natural killer cell-derived exosomal miR-3607-3p inhibits pancreatic cancer progression by targeting IL-26. Frontiers in Immunology, 10, 2819. https://doi.org/10.3389/fimmu.2019.02819
Chen, K., Cheng, M. P., Hammond, S. P., Einsele, H., & Marty, F. M. (2018). Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Advances, 2(16), 2159–2175.
GriffithsP, B., & ReevesM. (2015). The pathogenesis of human cytomegalovirus. The Journal of Pathology, 235(2), 288–297.
Camargo, J. F., & Komanduri, K. V. (2017). Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematology/Oncology and Stem Cell Therapy, 10(4), 233–238.
Foley, B., Cooley, S., Verneris, M. R., Pitt, M., Curtsinger, J., Luo, X., et al. (2012). Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C + natural killer cells with potent function. Blood, 119(11), 2665–2674.
Cichocki, F., Taras, E., Chiuppesi, F., Wagner, J. E., Blazar, B. R., Brunstein, C., Luo, X., Diamond, D. J., Cooley, S., Weisdorf, D. J., & Miller, J. S. (2019). Adaptive NK cell reconstitution is associated with better clinical outcomes. JCI Insight, 4(2), e125553. https://doi.org/10.1172/jci.insight.125553
Sun, J. C., Beilke, J. N., & Lanier, L. L. (2009). Adaptive immune features of natural killer cells. Nature, 457(7229), 557–561.
Cichocki, F., Cooley, S., Davis, Z., DeFor, T. E., Schlums, H., Zhang, B., et al. (2016). CD56 dim CD57 + NKG2C + NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia, 30(2), 456–463.
Foley, B., Cooley, S., Verneris, M. R., Curtsinger, J., Luo, X., Waller, E. K., et al. (2012). Human cytomegalovirus (CMV)-induced memory-like NKG2C + NK cells are transplantable and expand in vivo in response to recipient CMV antigen. The Journal of Immunology, 189(10), 5082–5088.
Muccio, L., Bertaina, A., Falco, M., Pende, D., Meazza, R., Lopez-Botet, M., et al. (2016). Analysis of memory-like natural killer cells in human cytomegalovirus-infected children undergoing αβ + T and B cell-depleted hematopoietic stem cell transplantation for hematological malignancies. Haematologica, 101(3), 371–381.
Béziat, V., Liu, L. L., Malmberg, J. A., Ivarsson, M. A., Sohlberg, E., Björklund, A. T., et al. (2013). NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood, 121(14), 2678–2688.
Lopez-Vergès, S., Milush, J. M., Schwartz, B. S., Pando, M. J., Jarjoura, J., York, V. A., et al. (2011). Expansion of a unique CD57 + NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proceedings of the National Academy of Sciences, 108(36), 14725-32.
Bélanger, S., Tu, M. M., Rahim, M. M. A., Mahmoud, A. B., Patel, R., Tai, L. H., et al. (2012). Impaired natural killer cell self-education and “missing-self” responses in Ly49-deficient mice. Blood, 120(3), 592–602.
Author information
Authors and Affiliations
Contributions
Alireza zafarani, Mahsa Taghavi-Farahabadi and Mansoure Mansouri wrote the manuscript, Mohammad Hossein Razizadeh, Mohammad Reza Amirzargar prepared the tables, Mohammad Mahmoudi prepared the figure, supervised the study and correspondence during the paper preparation and submission, RSV read and edited the final submission. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zafarani, A., Taghavi-Farahabadi, M., Razizadeh, M.H. et al. The Role of NK Cells and Their Exosomes in Graft Versus Host Disease and Graft Versus Leukemia. Stem Cell Rev and Rep 19, 26–45 (2023). https://doi.org/10.1007/s12015-022-10449-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10449-2