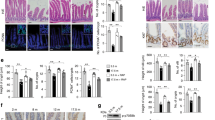
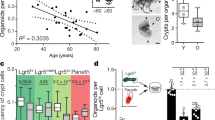
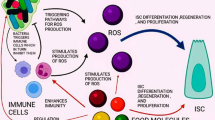
Abstract
The intestine integrates the function of digestion, absorption, and barrier, which is easily damaged by the external factors upon ageing. The intestinal stem cells (ISCs) exist at the intestinal crypt base and play an indispensable role in intestinal homeostasis and regeneration. The intestine ageing contributes to malabsorption and other associated illnesses, which were considered to be related to ISCs. Here, we summarize the current research progress of mammalian ISCs ageing and pay more attention to the central regulatory role of the mTORC1 signaling pathway in regulating mammalian ISCs ageing, and its related AMPK, FOXO, Wnt signaling pathways. Furthermore, we also discuss the interventions aimed at mTORC1 and its associated signaling pathways, which may provide potential strategies for rejuvenating aged ISCs and the therapy of age-related intestinal diseases.

Many signaling pathways are altered in the ageing ISCs, thereby inducing the decrease of ISC self-renewal, differentiation, and regeneration, an increasing of oxidative stress may contribute to damage to the ISCs. Interventions such as calorie restriction, fasting and so on can effectively alleviate these adverse effects.



Similar content being viewed by others
References
Barker, N., Van Es, J. H., Kuipers, J., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 449, 1003–1007.
Rizk, P., & Barker, N. (2012). Gut stem cells in tissue renewal and disease: Methods, markers, and myths. Wiley Interdisciplinary Reviews Systems Biology and Medicine, 4, 475–496.
Sangiorgi, E., & Capecchi, M. R. (2008). Bmi1 is expressed in vivo in intestinal stem cells. Nature Genetics, 40, 915–920.
Li, C. M., Yan, H. C., Fu, H. L., Xu, G. F., & Wang, X. Q. (2014). Molecular cloning, sequence analysis, and function of the intestinal epithelial stem cell marker Bmi1 in pig intestinal epithelial cells. Journal of Animal Science, 92(1), 85–94.
Li, X. G., Wang, Z., Chen, R. Q., Fu, H. L., Gao, C. Q., Yan, H. C., Xing, G. X., & Wang, X. Q. (2018). LGR5 and BMI1 increase pig intestinal epithelial cell proliferation by stimulating WNT/β-catenin signaling. International Journal of Molecular Science, 19(4), 1036.
Smith, N. R., Swain, J. R., Davies, P. S., Gallagher, A. C., Parappilly, M. S., Beach, C. Z., Streeter, P. R., Williamson, I. A., Magness, S. T., & Wong, M. H. (2018). Monoclonal antibodies reveal dynamic plasticity between Lgr5- and Bmi1-expressing intestinal cell populations. Cellular & Molecular Gastroenterology & Hepatology, 6(1), 79–96.
Yin, X. L., Farin, H. F., van Es, J. H., et al. (2014). Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nature Methods, 11, 106–112.
Li, X. G., Zhu, M., Chen, M. X., Fan, H. B., Fu, H. L., Zhou, J. Y., Zhai, Z. Y., Gao, C. Q., Yan, H. C., & Wang, X. Q. (2019). Acute exposure to deoxynivalenol inhibits porcine enteroid activity via suppression of the Wnt/β-catenin pathway. Toxicology Letter, 305, 19–31.
Liang, S. J., Li, X. G., & Wang, X. Q. (2019). Notch signaling in mammalian intestinal stem cells: Determining cell fate and maintaining homeostasis. Current Stem Cell Research & Therapy, 14(7), 583–590.
Zhou, J. Y., Wang, Z., Zhang, S. W., Lin, H. L., Gao, C. Q., Zhao, J. C., Yang, C., & Wang, X. Q. (2019). Methionine and its hydroxyl analogs improve stem cell activity to eliminate deoxynivalenol-induced intestinal injury by reactivating Wnt/β-catenin signaling. Journal of Agricultural and Food Chemistry, 67, 11464–11473.
Zhou, J. Y., Huang, D. G., Qin, Y. C., Li, X. G., Gao, C. Q., Yan, H. C., & Wang, X. Q. (2019). mTORC1 signaling activation increases intestinal stem cell activity and promotes epithelial cell proliferation. Journal of Cellular Physiology, 234(10), 19028–19038.
Zhou, J. Y., Zhang, S. W., Lin, H. L., Gao, C. Q., Yan, H. C., & Wang, X. Q. (2019). Hydrolyzed wheat gluten alleviates deoxynivalenol-induced intestinal injury by promoting intestinal stem cell proliferation and differentiation via upregulation of Wnt/β-catenin signaling in mice. Food and Chemical Toxicology, 131, 110579.
Zhou, J. Y., Lin, H. L., Wang, Z., Zhang, S. W., Huang, D. G., Gao, C. Q., Yan, H. C., & Wang, X. Q. (2020). Zinc L-aspartate enhances intestinal stem cell activity to protect the integrity of the intestinal mucosa against deoxynivalenol through activation of the Wnt/β-catenin signaling pathway. Environmental Pollution, 262, 114290.
Tian, H., Biehs, B., Chiu, C., Siebel, C. W., Wu, Y., Costa, M., de Sauvage, F. J., & Klein, O. D. (2015). Opposing activities of notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Reports, 11(1), 33–42.
Lukonin, I., Serra, D., Challet, M. L., et al. (2020). Phenotypic landscape of intestinal organoid regeneration. Nature, 586, 275–280.
McHugh, D., & Gil, J. (2017). Senescence and aging: Causes, consequences, and therapeutic avenues. Journal of Cell Biology, 217(1), 65–77.
Lópezotín, C., Blasco, M. A., Partridge, L., et al. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217.
Paradies, G., Petrosillo, G., Paradies, V., & Ruggiero, F. M. (2010). Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radical Biology & Medicine, 48(10), 1286–1295.
O'Sullivan, R. J., & Karlseder, J. (2010). Telomeres: Protecting chromosomes against genome instability. Nature Reviews Molecular Cell Biology, 11, 171–181.
Schepers, A. G., Vries, R., van den Born, M., et al. (2014). Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO Journal, 30(6), 1104–1109.
Martin, K., Kirkwood, T. B. L., & Potten, C. S. (1998). Age changes in stem cells of murine small intestinal crypts. Clinical Science, 241(2), 316–323.
Ren, W. Y., Wu, K. F., Li, X., Luo, M., Liu, H. C., Zhang, S. C., & Hu, Y. (2014). Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clinical & Experimental Research, 26(2), 183–191.
Gebert, N., Cheng, C. W., Kirkpatrick, J. M., di Fraia, D., Yun, J., Schädel, P., Pace, S., Garside, G. B., Werz, O., Rudolph, K. L., Jasper, H., Yilmaz, Ö. H., & Ori, A. (2020). Region-specific proteome changes of the intestinal epithelium during aging and dietary restriction. Cell Reports, 31(4), 107565.
Mabbott, N. A. (2015). A breakdown in communication? Understanding the effects of aging on the human small intestine epithelium. Clinical Science, 129(7), 529–531.
Man, A. L., Bertelli, E., Rentini, S., Regoli, M., Briars, G., Marini, M., Watson, A. J. M., & Nicoletti, C. (2015). Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clinical Science, 129(7), 515–527.
Woudstra, T., & Thomson, A. B. R. (2002). Nutrient absorption and intestinal adaptation with ageing. Best Practice & Research Clinical Gastroenterology, 16(1), 1–15.
Yamamoto, K., Kitano, Y., Shuang, E., et al. (2014). Decreased lipid absorption due to reduced pancreatic lipase activity in aging male mice. Biogerontology., 15(5), 463–473.
Schultz, M. B., & Sinclair, D. A. (2016). When stem cells grow old: Phenotypes and mechanisms of stem cell aging. Development, 143(1), 3–14.
Moorefield, E. C., Andres, S. F., Eric, B. R., et al. (2017). Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging, 9, 1898–1915.
He, D., Wu, H., Xiang, J., et al. (2020). Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nature Communications, 11(1), 397–408.
Jiahn, C., Nikolai, R., Poornima, G., et al. (2018). Intestinal crypts recover rapidly from focal damage with coordinated motion of stem cells that is impaired by aging. Scientific Reports, 8(1), 10989.
Lewis, S. K., Nachun, D., Martin, M. G., Horvath, S., Coppola, G., & Jones, D. L. (2020). DNA methylation analysis validates Organoids as a viable model for studying human intestinal aging. Cellular and Molecular Gastroenterology and Hepatology, 9(3), 527–541.
Martin, K., Potten, C. S., Roberts, S. A., et al. (1998). Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. Journal of Cell Science, 111(16), 2297–2303.
Sandström, O., & El-Salhy, M. (1999). Ageing and endocrine cells of human duodenum. Mechanisms of Ageing & Development, 108(1), 39–48.
Sandström, O., Mahdavi, J., & El-Salhy, M. (1998). Effect of ageing on colonic endocrine cell population in mouse. Gerontology, 44(6), 324–330.
Zhu, M., & Wang, X. Q. (2020). Regulation of mTORC1 by small GTPases in response to nutrients. The Journal of Nutrition, 150(5), 1004–1011.
Sampson, L. L., Davis, A. K., Grogg, M. W., & Zheng, Y. (2016). mTOR disruption causes intestinal epithelial cell defects and intestinal atrophy postinjury in mice. FASEB Journal, 30(3), 1263–1275.
Zhu, M., Qin, Y. C., Gao, C. Q., Yan, H. C., Li, X. G., & Wang, X. Q. (2019). Extracellular glutamate-induced mTORC1 activation via the IR/IRS/PI3K/Akt pathway enhances the expansion of porcine intestinal stem cells. Journal of Agricultural & Food Chemistry, 67(34), 9510–9521.
Zhu, M., Qin, Y. C., Gao, C. Q., Yan, H. C., & Wang, X. Q. (2020). L-glutamate drives porcine intestinal epithelial renewal by increasing stem cell activity via upregulation of the EGFR-ERK-mTORC1 pathway. Food & Function, 11(3), 2714–2724.
Johnson, S. C., Rabinovitch, P. S., & Kaeberlein, M. (2013). mTOR is a key modulator of ageing and age-related disease. Nature, 493(7432), 338–345.
de Oliveira, B. M., Cirilo, C. P., de Santi-Rampazzo, A. P., et al. (2015). Intestinal morphology adjustments caused by dietary restriction improves the nutritional status during the aging process of rats. Experimental Gerontology, 69, 85–93.
Garratt, M., Nakagawa, S., & Simons, M. J. P. (2016). Comparative idiosyncrasies in life extension by reduced mTOR signalling and its distinctiveness from dietary restriction. Aging Cell, 15(4), 737–743.
Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., Nadon, N. L., Wilkinson, J. E., Frenkel, K., Carter, C. S., Pahor, M., Javors, M. A., Fernandez, E., & Miller, R. A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392–395.
Fontana, L., & Partridge, L. (2015). Promoting health and longevity through diet, from model organisms to humans. Cell, 161(1), 106–118.
Leonie, K. H., & Eric, R. (2003). Calorie restriction and aging: Review of the literature and implications for studies in humans. American Journal of Clinical Nutrition, 78(3), 361–369.
Yilmaz, Ö. H., Katajisto, P., Lamming, D. W., Gültekin, Y., Bauer-Rowe, K. E., Sengupta, S., Birsoy, K., Dursun, A., Yilmaz, V. O., Selig, M., Nielsen, G. P., Mino-Kenudson, M., Zukerberg, L. R., Bhan, A. K., Deshpande, V., & Sabatini, D. M. (2012). mTORC1 in the Paneth cell niche couples intestinal stem cell function to calorie intake. Nature, 486(7404), 490–495.
Igarashi, M., & Guarente, L. (2016). mTORC1 and SIRT1 cooperate to Foster expansion of gut adult stem cells during calorie restriction. Cell, 166(2), 436–450.
Yousefi, M., Nakauka-Ddamba, A., Berry, C. T., Li, N., Schoenberger, J., Simeonov, K. P., Cedeno, R. J., Yu, Z., & Lengner, C. J. (2018). Calorie restriction governs intestinal epithelial regeneration through cell-autonomous regulation of mTORC1 in reserve stem cells. Stem Cell Reports, 10(3), 703–711.
Dan, N., Samson, S. L., Reddy, V. T., et al. (2013). Impaired mitochondrial fatty acid oxidation and insulin resistance in aging, novel protective role of glutathione. Aging Cell, 12(3), 415–425.
Mihaylova, M. M., Cheng, C. W., Cao, A. Q., Tripathi, S., Mana, M. D., Bauer-Rowe, K. E., Abu-Remaileh, M., Clavain, L., Erdemir, A., Lewis, C. A., Freinkman, E., Dickey, A. S., la Spada, A. R., Huang, Y., Bell, G. W., Deshpande, V., Carmeliet, P., Katajisto, P., Sabatini, D. M., & Yilmaz, Ö. H. (2018). Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell, 22(5), 769–778.
Richmond, C. A., Shah, M. S., Deary, L. T., et al. (2015). Dormant intestinal stem cells are regulated by PTEN and nutritional status. Cell Reports, 3(11), 2403–2411.
Song, M. S., Salmena, L., & Pandolfi, P. P. (2012). The functions and regulation of the PTEN tumour suppressor. Nature Reviews Molecular Cell Biology, 13(5), 8136–8147.
Gwinn, D. M., Shackelford, D. B., Egan, D. F., Mihaylova, M. M., Mery, A., Vasquez, D. S., Turk, B. E., & Shaw, R. J. (2008). AMPK phosphorylation of raptor 375 mediates a metabolic checkpoint. Molecular Cell, 30(2), 214–226.
Cantó, C., Gerhart-Hines, Z., Feige, J., et al. (2009). AMPK regulates energy expenditure by modulating NAD+metabolism and SIRT1 activity. Nature, 458, 1056–1060.
Carling, D. (2004). The AMP-activated protein kinase cascade – A unifying system for energy control. Trends in Biochemical Sciences, 29(1), 18–24.
Guarente, L. (2013). Calorie restriction and sirtuins revisited. Genes & Development, 27(19), 2072–2085.
Wang, Y., Liang, Y., & Vanhoutte, P. M. (2011). SIRT1 and AMPK in regulating mammalian senescence: A critical review and a working model. FEBS Letters, 585(7), 986–994.
Kanfi, Y., Naiman, S., Amir, G., Peshti, V., Zinman, G., Nahum, L., Bar-Joseph, Z., & Cohen, H. Y. (2012). The sirtuin SIRT6 regulates lifespan in male mice. Nature, 483(7388), 218–221.
Mitchell, S. J., Martin-Montalvo, A., Mercken, E. M., Palacios, H. H., Ward, T. M., Abulwerdi, G., Minor, R. K., Vlasuk, G. P., Ellis, J. L., Sinclair, D. A., Dawson, J., Allison, D. B., Zhang, Y., Becker, K. G., Bernier, M., & de Cabo, R. (2014). The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Reports, 6(5), 836–843.
Satoh, A., Brace, C. S., Ben-Josef, G., West, T., Wozniak, D. F., Holtzman, D. M., Herzog, E. D., & Imai, S. I. (2010). SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. Journal of Neuroscience, 30, 10220–10232.
Igarashi, M., Miura, M., Williams, E. O., et al. (2019). NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell, 18(3), e12935.
Uchida, R., Saito, Y., Nogami, K., Kajiyama, Y., Suzuki, Y., Kawase, Y., Nakaoka, T., Muramatsu, T., Kimura, M., & Saito, H. (2018). Epigenetic silencing of Lgr5 induces senescence of intestinal epithelial organoids during the process of aging. NPJ Aging Mechanisms and Disease, 4, 12.
Bonkowski, M. S., & Sinclair, D. A. (2016). Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nature Reviews Molecular Cell Biology, 17(11), 679–690.
Eijkelenboom, A., & Burgering, B. M. T. (2013). FOXOs: Signalling integrators for homeostasis maintenance. Nature Reviews Molecular Cell Biology, 14(2), 83–97.
Martins R., Lithgow G .J. and Link W. (2016). Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell, 15(2), 196–207.
Kim, D. H., Park, M. H., Lee, E. K., Choi, Y. J., Chung, K. W., Moon, K. M., Kim, M. J., An, H. J., Park, J. W., Kim, N. D., Yu, B. P., & Chung, H. Y. (2015). The roles of FoxOs in modulation of aging by calorie restriction. Biogerontology, 16, 1–14.
Yamaza, H., Komatsu, T., Wakita, S., et al. (2010). FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell, 36(1), 372–382.
Shimokawa, I., Komatsu, T., Hayashi, N., Kim, S. E., Kawata, T., Park, S., Hayashi, H., Yamaza, H., Chiba, T., & Mori, R. (2015). The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell, 14(4), 707–709.
Tothova, Z., Kollipara, R., Huntly, B. J., Lee, B. H., Castrillon, D. H., Cullen, D. E., McDowell, E. P., Lazo-Kallanian, S., Williams, I. R., Sears, C., Armstrong, S. A., Passegué, E., DePinho, R. A., & Gilliland, D. G. (2007). FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell, 128(2), 325–339.
Cirilo, C. P., Schoffen, J., De Santi-Rampazzo, A. P., et al. (2013). Dietary restriction interferes with oxidative status and intrinsic intestinal innervation in aging rats. Nutrition, 29(4), 673–680.
Grattagliano, I., Portincasa, P., Cocco, T., Moschetta, A., di Paola, M., Palmieri, V. O., & Palasciano, G. (2004). Effect of dietary restriction and N-acetylcysteine supplementation on intestinal mucosa and liver mitochondrial redox status and function in aged rats. Experimental Gerontology, 39(9), 1323–1332.
Liu, L., & Rando, T. A. (2011). Manifestations and mechanisms of stem cell aging. Journal of Cell Biology, 193(2), 257–266.
Zhou, J. Y., Huang, D. G., Zhu, M., Gao, C. Q., Yan, H. C., Li, X. G., & Wang, X. Q. (2020). Wnt/β-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell expansion through endoplasmic reticulum stress. Journal of Cellular Physiology, 235, 5613–5627.
Fan, H. B., Zhai, Z. Y., Li, X. G., Gao, C. Q., Yan, H. C., Chen, Z. S., & Wang, X. Q. (2017). CDX2 stimulates the proliferation of porcine intestinal epithelial cells by activating the mTORC1 and Wnt/β-catenin signaling pathways. International Journal of Molecular Sciences, 18(11), 2447.
Tao, S., Tang, D., Morita, Y., Sperka, T., Omrani, O., Lechel, A., Sakk, V., Kraus, J., Kestler, H. A., Kühl, M., & Rudolph, K. L. (2015). Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. EMBO Journal, 34(5), 624–640.
Nalapareddy, K., Nattamai, K. J., Kumar, R. S., et al. (2018). Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Reports, 18(11), 2608–2621.
Cui, H., Tang, D., Garside, G. B., Zeng, T., Wang, Y., Tao, Z., Zhang, L., & Tao, S. (2019). Wnt signaling mediates the aging-induced differentiation impairment of intestinal stem cells. Stem Cell Reviews and Reports, 15(3), 448–455.
Pentinmikko, N., Iqbal, S., Mana, M., Andersson, S., Cognetta III, A. B., Suciu, R. M., Roper, J., Luopajärvi, K., Markelin, E., Gopalakrishnan, S., Smolander, O. P., Naranjo, S., Saarinen, T., Juuti, A., Pietiläinen, K., Auvinen, P., Ristimäki, A., Gupta, N., Tammela, T., Jacks, T., Sabatini, D. M., Cravatt, B. F., Yilmaz, Ö. H., & Katajisto, P. (2019). Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature, 571, 398–402.
Kakugawa, S., Langton, P. F., Zebisch, M., Howell, S. A., Chang, T. H., Liu, Y., Feizi, T., Bineva, G., O’Reilly, N., Snijders, A. P., Jones, E. Y., & Vincent, J. P. (2015). Notum deacylates Wnt proteins to suppress signalling activity. Nature, 519(7542), 187–192.
Cruciat, C. M., & Niehrs, C. (2013). Secreted and Transmembrane Wnt inhibitors and activators. Cold Spring Harbor Perspectives in Biology, 5(3), 313–314.
Ashton, G. H., Morton, J. P., Myant, K., et al. (2010). Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Developmental Cell, 2, 259–269.
Pan, H., & Finkel, T. (2017). Key proteins and pathways that regulate lifespan. Journal of Biological Chemistry, 292(16), 6452–6460.
Conboy, I. M., Conboy, M. J., Smythe, G. M., & Rando, T. A. (2003). Notch-mediated restoration of regenerative potential to aged muscle. Science, 302(5650), 1575–1577.
Renault, V. M., Rafalski, V. A., Morgan, A. A., et al. (2009). FoxO3 Regulates Neural Stem Cell Homeostasis. Cell Stem Cell, 5(5), 540–553.
Myant, K. B., Cammareri, P., Mcghee, E. J., et al. (2013). ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal Cancer initiation. Cell Stem Cell, 12(6), 761–773.
Paul, M. K., Bisht, B., Darmawan, D. O., et al. (2014). Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent notch signaling. Cell Stem Cell, 5(2), 199–214.
O'Toole, P. W., & Jeffery, I. B. (2015). Gut microbiota and aging. Science, 350(6265), 1214–1215.
Thevaranjan, N., Puchta, A., & Schulz, C. (2018). Age-associated microbial Dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host & Microbe, 23(4), 455–466.
Deng, F. L., Li, Y., & Zhao, J. C. (2019). The gut microbiome of healthy long-living people. Aging, 11(2), 289–290.
Sivamaruthi, B. S., Kesika, P., & Chaiyasut, C. (2018). A review on anti-aging properties of probiotics. International Journal of Applied Pharmaceutics, 10(5), 23–27.
Martinez-Jimenez, C. P., Eling, N., Chen, H. C., Vallejos, C. A., Kolodziejczyk, A. A., Connor, F., Stojic, L., Rayner, T. F., Stubbington, M. J. T., Teichmann, S. A., de la Roche, M., Marioni, J. C., & Odom, D. T. (2017). Aging increases cell-to-cell transcriptional variability upon immune stimulation. Science, 355(6332), 1433–1436.
Wang, S., Zheng, Y., Li, J., Yu, Y., Zhang, W., Song, M., Liu, Z., Min, Z., Hu, H., Jing, Y., He, X., Sun, L., Ma, L., Esteban, C. R., Chan, P., Qiao, J., Zhou, Q., Izpisua Belmonte, J. C., Qu, J., Tang, F., & Liu, G. H. (2020). Single-cell Transcriptomic atlas of primate ovarian aging. Cell, 180(3), 585–600.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFD0500501), the National Natural Science Foundation of China (31872389, 32072777), the Basic and Applied Basic Research Foundation of Guangdong Province (2019B1515210021).
Author information
Authors and Affiliations
Contributions
Xiu-qi Wang had the idea for the article, Shao-jie Liang performed the literature search and finished the manuscript, Jia-yi Zhou critically revised the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, Sj., Zhou, Jy. & Wang, Xq. Signaling Network Centered on mTORC1 Dominates Mammalian Intestinal Stem Cell Ageing. Stem Cell Rev and Rep 17, 842–849 (2021). https://doi.org/10.1007/s12015-020-10073-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10073-y