Disease is the retribution of outraged Nature.
Hosea Ballou.
Abstract
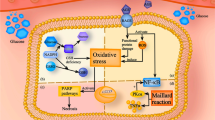
Diabetic neuropathy (DN) represents the main cause of morbidity and mortality among diabetic patients. Clinical data support the conclusion that the severity of DN is related to the frequency and duration of hyperglycemic periods. The presented experimental and clinical evidences propose that changes in cellular function resulting in oxidative stress act as a leading factor in the development and progression of DN. Hyperglycemia- and dyslipidemia-driven oxidative stress is a major contributor, enhanced by advanced glycation end product (AGE) formation and polyol pathway activation. There are several polymorphous pathways that lead to oxidative stress in the peripheral nervous system in chronic hyperglycemia. This article demonstrates the origin of oxidative stress derived from glycation reactions and genetic variations within the antioxidant genes which could be implicated in the pathogenesis of DN. In the diabetic state, unchecked superoxide accumulation and resultant increases in polyol pathway activity, AGEs accumulation, protein kinase C activity, and hexosamine flux trigger a feed-forward system of progressive cellular dysfunction. In nerve, this confluence of metabolic and vascular disturbances leads to impaired neural function and loss of neurotrophic support, and over the long term, can mediate apoptosis of neurons and Schwann cells, the glial cells of the peripheral nervous system. In this article, we consider AGE-mediated reactive oxygen species (ROS) generation as a pathogenesis factor in the development of DN. It is likely that oxidative modification of proteins and other biomolecules might be the consequence of local generation of superoxide on the interaction of the residues of l-lysine (and probably other amino acids) with α-ketoaldehydes. This phenomenon of non-enzymatic superoxide generation might be an element of autocatalytic intensification of pathophysiological action of carbonyl stress. Glyoxal and methylglyoxal formed during metabolic pathway are detoxified by the glyoxalase system with reduced glutathione as co-factor. The concentration of reduced glutathione may be decreased by oxidative stress and by decreased in situ glutathione reductase activity in diabetes mellitus. Genetic variations within the antioxidant genes therefore could be implicated in the pathogenesis of DN. In this work, the supporting data about the association between the −262T > C polymorphism of the catalase (CAT) gene and DN were shown. The −262TT genotype of the CAT gene was significantly associated with higher erythrocyte catalase activity in blood of DN patients compared to the −262CC genotype (17.8 ± 2.7 × 104 IU/g Hb vs. 13.5 ± 3.2 × 104 IU/g Hb, P = 0.0022). The role of these factors in the development of diabetic complications and the prospective prevention of DN by supplementation in formulations of transglycating imidazole-containing peptide-based antioxidants (non-hydrolyzed carnosine, carcinine, n-acetylcarcinine) scavenging ROS in the glycation reaction, modifying the activity of enzymic and non-enzymic antioxidant defenses that participate in metabolic processes with ability of controlling at transcriptional levels the differential expression of several genes encoding antioxidant enzymes inherent to DN in Type I Diabetic patients, now deserve investigation.





Similar content being viewed by others
References
Windebank, A. J., & Feldman, E. L. (2001). Diabetes and the nervous system. In M. J. Aminoff (Ed.), Neurology and general medicine (pp. 341–364). Philadelphia, PA: Churchill Livingstone.
Brownlee, M. (2001). Biochemistry and molecular cell biology of diabetic complications. Nature, 414(6865), 813–820.
Feldman, E. L. (2003). Oxidative stress and diabetic neuropathy: A new understanding of an old problem. The Journal of Clinical Investigation, 111(4), 431–433.
Gardner, T. W., Antonetti, D. A., Barber, A. J., LaNoue, K. F., & Levison, S. W. (2002). Diabetic retinopathy: More than meets the eye. Survey of Ophthalmology, 47(Suppl 2), S253–S262.
Fong, D. S., Aiello, L., Gardner, T. W., King, G. L., Blankenship, G., Cavallerano, J. D., et al. (2003). Diabetic retinopathy. Diabetes Care, 26(1), 226–229.
Fong, D. S., Aiello, L., Gardner, T. W., King, G. L., Blankenship, G., Cavallerano, J. D., et al. (2003). Diabetic retinopathy. Diabetes Care, 26(Suppl 1), S99–S102.
Skyler, J. S. (2001). Microvascular complications. Retinopathy and nephropathy. Endocrinology and Metabolism Clinics of North America, 30(4), 833–856.
Calcutt, N. A. (2002). Potential mechanisms of neuropathic pain in diabetes. International Review of Neurobiology, 50, 205–228.
Feldman, E. L., Stevens, M. J., & Russell, J. W. (2002). Diabetic peripheral and autonomic neuropathy. In M. A. Sperling (Ed.), Contemporary endocrinology (pp. 437–461). Totowa, NJ: Humana Press.
Feldman, E. L., Stevens, M. J., Russell, J. W., & Greene, D. A. (2002). Somatosensory neuropathy. In D. Porte Jr, R. S. Sherwin, & A. Baron (Eds.), Ellenberg and Rifkin’s diabetes mellitus (pp. 771–788). New York: McGraw Hill.
Vinik, A. I. (2002). Diabetic autonomic neuropathy. In D. Porte Jr, R. S. Sherwin, & A. Baron (Eds.), Ellenberg and Rifkin’s diabetes mellitus (pp. 789–804). New York: McGraw Hill.
Feldman, E. L., Stevens, M. J., Russell, J. W., & Greene, D. A. (2001). Diabetic neuropathy. In K. L. Becker (Ed.), Principles and practice of endocrinology and metabolism (pp. 1391–1399). Philadelphia, PA: Lippincott Williams & Wilkins.
Dyck. P. J., Kratz, K. M., Karnes, J. L., Litchy, W. J., Klein, R., Pach, J. M., et al. (1993). The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology, 43(4), 817–824. Erratum in: Neurology 1993; 43(11):2345.
Dyck, P. J., Giannini, C. (1996). Pathologic alterations in the diabetic neuropathies of humans: A review. Journal of Neuropathology and Experimental Neurology, 55(12), 1181–1193. Comment in: Journal of Neuropathology and Experimental Neurology 1997; 56(4):458.
Boel, E., Selmer, J., Flodgaard, H. J., & Jensen, T. (1995). Diabetic late complications: Will aldose reductase inhibitors or inhibitors of advanced glycosylation endproduct formation hold promise? Journal of Diabetes and Its Complications, 9(2), 104–129.
Poduslo, J. F., & Curran, G. L. (1992). Increased permeability across the blood–nerve barrier of albumin glycated in vitro and in vivo from patients with diabetic polyneuropathy. Proceedings of the National Academy of Science of the USA, 89(6), 2218–2222.
Sugimoto, K., Nishizawa, Y., Horiuchi, S., & Yagihashi, S. (1997). Localization in human diabetic peripheral nerve of N(epsilon)-carboxymethyllysine-protein adducts, an advanced glycation endproduct. Diabetologia, 40, 1380–1387.
Graham, A. R., & Johnson, P. C. (1985). Direct immunofluorescence findings in peripheral nerve from patients with diabetic neuropathy. Annals of Neurology, 17, 450–454.
Obrosova, I. G. (2003). Update on the pathogenesis of diabetic neuropathy. Current Diabetes Reports, 3(6), 439–445.
Stevens, M. J., Obrosova, I., Pop-Busui, R., Greene, D. A., & Feldman, E. L. (2002). Pathogenesis of diabetic neuropathy. In D. Porte Jr, R. S. Sherwin, & A. Baron (Eds.), Ellenberg and Rifkin’s diabetes mellitus (pp. 747–770). New York: McGraw Hill.
Greene, D. A., Obrosova, I., Stevens, M. J., & Feldman, E. L. (2000). Pathways of glucose-mediated oxidative stress in diabetic neuropathy. In L. Packer, P. Rosen, H. J. Tritschler, G. L. King, & A. Azzi (Eds.), Antioxidants in diabetes management (pp. 111–119). New York: Marcel Dekker Inc.
Cameron, N. E., Eaton, S. E., Cotter, M. A., & Tesfaye, S. (2001). Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia, 44(11), 1973–1988.
Russell, J. W., Sullivan, K. A., Windebank, A. J., Herrmann, D. N., & Feldman, E. L. (1999). Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiology of Disease, 6(5), 347–363.
Russell, J. W., Golovoy, D., Vincent, A. M., Mahendru, P., Olzmann, J. A., Mentzer, A., & Feldman, E. L. (2002). High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB Journal, 16(13), 1738–1748.
Schmeichel, A. M., Schmelzer, J. D., & Low, P. A. (2003). Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes, 52(1), 165–171.
Apfel, S. C. (1999). Neurotrophic factors and diabetic peripheral neuropathy. European Neurology, 41(Suppl. 1), 27–34.
Tomlinson, D. R., Fernyhough, P., & Diemel, L. T. (1997). Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes, 1997(46), S43–S49.
Feldman, E. L., & Windebank, A. J. (1998). Growth factors and peripheral neuropathy. In P. J. Dyck & P. K. Thomas (Eds.), Diabetic neuropathy (pp. 377–386). Philadelphia, PA: W.B. Saunders Co.
Polydefkis, M., Griffin, J. W., & McArthur, J. (2003). New insights into diabetic polyneuropathy. JAMA, 290(10), 1371–1376.
Apfel, S. C. (1999). Nerve regeneration in diabetic neuropathy. Diabetes, Obesity & Metabolism, 1, 3–11.
Vinik, A. I. (1999). Diabetic neuropathy: Pathogenesis and therapy. American Journal of Medicine, 107(2B), 17S–26S.
Folmer, V., Soares, J. C., & Rocha, J. B. (2002). Oxidative stress in mice is dependent on the free glucose content of the diet. International Journal of Biochemistry & Cell Biology, 34, 1279–1285.
Thornalley, P. J. (2002). Glycation in diabetic neuropathy: Characteristics, consequences, causes, and therapeutic options. International Review of Neurobiology, 50, 37–57.
Cameron, N. E., & Cotter, M. A. (1995). Neurovascular dysfunction in diabetic rats. Potential contribution of autoxidation and free radicals examined using transition metal chelating agents. The Journal of Clinical Investigation, 96, 1159–1163.
Singh, R., Barden, A., Mori, T., & Beilin, L. (2001). Advanced glycation end-products: A review. Diabetologia, 44, 129–146.
Lander, H. M., Tauras, J. M., Ogiste, J. S., Hori, O., Moss, R. A., & Schmidt, A. M. (1997). Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. Journal of Biological Chemistry, 272, 17810–17814.
Wautier, J. L., Wautier, M. P., Schmidt, A. M., Anderson, G. M., Hori, O., Zoukourian, C., et al. (1994). Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: A link between surface-associated AGEs and diabetic complications. Proceedings of the National Academy of Sciences of the USA, 91, 7742–7746.
Yan, S. D., Schmidt, A. M., Anderson, G. M., Zhang, J., Brett, J., Zou, Y. S., et al. (1994). Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. Journal of Biological Chemistry, 269(13), 9889–9897.
Wautier, M. P., Chappey, O., Corda, S., Stern, D. M., Schmidt, A. M., & Wautier, J. L. (2001). Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. American Journal of Physiology, 280, E685–E694.
Monnier, V. M., Vishwanath, V., Frank, K. E., Elmets, C. A., Dauchot, P., & Kohn, R. R. (1986). Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. New England Journal of Medicine, 314(7), 403–408.
Monnier, V. M., Kohn, R. R., & Cerami, A. (1984). Accelerated age-related browning of human collagen in diabetes mellitus. Proceedings of the National Academy of Sciences of the USA, 81(2), 583–587.
Vlassara, H., Brownlee, M., & Cerami, A. (1981). Nonenzymatic glycosylation of peripheral nerve protein in diabetes mellitus. Proceedings of the National Academy of Sciences of the USA, 78(8), 5190–5192.
Vlassara, H., Brownlee, M., & Cerami, A. (1983). Excessive nonenzymatic glycosylation of peripheral and central nervous system myelin components in diabetic rats. Diabetes, 32(7), 670–674.
Hicks, M., Delbridge, L., Yue, D. K., & Reeve, T. S. (1988). Catalysis of lipid peroxidation by glucose and glycosylated collagen. Biochemical and Biophysical Research Communications, 151(2), 649–655.
Bucala, R., Tracey, K. J., & Cerami, A. (1991). Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. The Journal of Clinical Investigation, 87(2), 432–438.
Simpson, J. A., Narita, S., Gieseg, S., Gebicki, S., Gebicki, J. M., & Dean, R. T. (1992). Long-lived reactive species on free-radical-damaged proteins. Biochemical Journal, 282(Pt 3), 621–624.
Brownlee, M., Vlassara, H., & Cerami, A. (1984). Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Annals of Internal Medicine, 101(4), 527–537.
Mullarkey, C. J., Edelstein, D., & Brownlee, M. (1990). Free radical generation by early glycation products: A mechanism for accelerated atherogenesis in diabetes. Biochemical and Biophysical Research Communications, 173(3), 932–939.
Chistiakov, D. A., Zotova, E. V., Savost’anov, K. V., Bursa, T. R., Galeev, I. V., Strokov, I. A., & Nosikov, V. V. (2006). The 262T > C promoter polymorphism of the catalase gene is associated with diabetic neuropathy in type 1 diabetic Russian patients. Diabetes & Metabolism, 32(1), 63–68.
Hermenegildo, C., Raya, A., Roma, J., & Romero, F. J. (1993). Decreased glutathione peroxidase activity in sciatic nerve of alloxan-induced diabetic mice and its correlation with blood glucose levels. Neurochemical Research, 18, 893–896.
Van Dam, P. S., van Asbeck, B. S., Bravenboer, B., van Oirschot, J. F. L. M., Gispen, W. H., & Marx, J. J. M. (1998). Nerve function and oxidative stress in diabetic and vitamin E-deficient rats. Free Radical Biology and Medicine, 24, 18–26.
Martin-Gallan, P., Carrascosa, A., Gussinye, M., & Dominguez, C. (2003). Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radical Biology and Medicine, 34, 1563–1574.
Merzouk, S., Hichami, A., Madani, S., Merzouk, H., Berrouiguet, A. Y., Prost, J., et al. (2003). Antioxidant status and levels of different vitamins determined by high performance liquid chromatography in diabetic subjects with multiple complications. General Physiology and Biophysics, 22(1), 15–27.
Yan, H., & Harding, J. J. (1997). Glycation-induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochemical Journal, 328, 599–605.
Morgan, P. E., Dean, R. T., & Davies, M. J. (2002). Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Archives of Biochemistry and Biophysics, 403, 259–269.
Requena, J. R., Fu, M. X., Ahmed, M. U., Jenkins, A. J., Lyons, T. J., Baynes, J. W., & Thorpe, S. R. (1997). Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochemical Journal, 322(Pt 1), 317–325.
Thornalley, P. J. (1985). Monosaccharide autoxidation in health and disease. Environmental Health Perspectives, 64, 297–307.
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.
(2003). Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care, 26(suppl 1), S5–S20.
Statement, Consensus. (1988). Report and recommendations of the San Antonio on diabetic neuropathy. American Diabetes Association American Academy of Neurology. Diabetes Care, 11, 592–597.
Bentler, E., Duron, O., & Kelly, B. M. (1963). Improved method for the determination of blood glutathione. Journal of Laboratory and Clinical Medicine, 61, 882–888.
Bentler, E. (Ed.). (1975). Red cell metabolism. A manual of biochemical methods. New York: Grune & Stratton.
Folsberg, E., de Faire, U., & Morgenstern, R. (1999). Low yield of polymorphisms from EST Blast searching: Analysis of genes related to oxidative stress and verification of the P197L polymorphism in GPX1. Human Mutation, 13, 294–300.
Folsberg, L., Lyrenas, L., de Faire, U., & Morgenstern, R. (2001). A common functional C-T substitution polymorphisms in the promoter region of the human catalase gene influences transcription factor binding, reported gene transcription and is correlated to blood catalase levels. Free Radical Biology and Medicine, 30, 500–505.
Thorpe, S. R., & Baynes, J. W. (2003). Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids, 25(3–4), 275–281. Epub 2003 Jul 29.
Bourajjaj, M., Stehouwer, C. D., van Hinsbergh, V. W., & Schalkwijk, C. G. (2003). Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochemical Society Transactions, 31(Pt 6), 1400–1402.
Thornalley, P. J. (1993). The glyoxalase system in health and disease. Molecular Aspects of Medicine, 14(4), 287–371.
Lo, T. W., Westwood, M. E., McLellan, A. C., Selwood, T., & Thornalley, P. J. (1994). Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. Journal of Biological Chemistry, 269(51), 32299–32305.
Suji, G., & Sivakami, S. (2007). DNA damage during glycation of lysine by methylglyoxal: Assessment of vitamins in preventing damage. Amino Acids, 33(4), 615–621. Epub 2007 Feb 16.
Yim, H. S., Kang, S. O., Hah, Y. C., Chock, P. B., & Yim, M. B. (1995). Free radicals generated during the glycation reaction of amino acids by methylglyoxal. A model study of protein-cross-linked free radicals. Journal of Biological Chemistry, 270(47), 28228–28233.
Shumaev, K. B., Gubkina, S. A., Kumskova, E. M., Shepelkova, G. S., Ruuge, E. K., & Lankin, V. Z. (2009). Superoxide formation as a result of interaction of l-lysine with dicarbonyl compounds and its possible mechanism. Biochemistry (Mosc), 74(4), 461–466.
McLaughlin, J. A., Pethig, R., & Szent-Györgyi, A. (1980). Spectroscopic studies of the protein-methylglyoxal adduct. Proceedings of the National Academy of Sciences of the USA, 77(2), 949–951.
Tarpey, M. M., Wink, D. A., & Grisham, M. B. (2004). Methods for detection of reactive metabolites of oxygen and nitrogen: In vitro and in vivo considerations. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 286(3), R431–R444.
Bisby, R. H., & Parker, A. W. (1991). Reactions of the alpha-tocopheroxyl radical in micellar solutions studied by nanosecond laser flash photolysis. FEBS Letters, 290(1–2), 205–208.
Fernyhough, P., & Jonathan, M. (2014). Mechanisms of disease: Mitrochondrial dysfunction in sensory neuropathy and other complications in diabetes. Handbook of Clinical Neurology, 126, 353–377.
Choi, J., Chandrasekaran, K., Inoue, T., Muragundla, A., Russell, J. W. (2014). PGC-1α regulation of mitochondrial degeneration in experimental diabetic neuropathy. Neurobiology of Disease, 64, 118–130.
Mizisin, A. P. (2014). Mechanisms of diabetic neuropathy: Schwann cells. Handbook of Clinical Neurology, 126, 401–428.
Xie, Z. X., Xia, S. F., Qiao, Y., Shi, Y. H., & Le, G. W. (2014). Effect of GABA on oxidative stress in the skeletal muscles and plasma free amino acids in mice fed high-fat diet. Journal of Animal Physiology and Animal Nutrition. doi:10.1111/jpn.12254.
Tsai, E. C., Hirsch, I. B., Brunzell, J. D., & Chait, A. (1994). Reduced plasma peroxyl radical trapping capacity and increased susceptibility of LDL to oxidation in poorly controlled IDDM. Diabetes, 43, 1010–1014.
Altomare, E., Vendemiale, G., Chicco, D., Procacci, V., & Cirelli, F. (1992). Increased lipid peroxidation in type 2 poorly controlled diabetic patients. Diabete et Metabolisme, 18, 264–271.
Zaltzberg, H., Kanter, Y., Aviram, M., & Levy, Y. (1999). Increased plasma oxidizability and decreased erythrocyte and plasma antioxidative capacity in patients with NIDDM. The Israel Medical Association Journal, 1, 228–231.
Sundaram, R. K., Bhaskar, A., Vijayalingam, S., Viswanathan, M., Mohan, R., & Shanmugasundaram, K. R. (1996). Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clinical Science (Lond), 90, 255–260.
Elhadd, T. A., Kennedy, G., Hill, A., McLaren, M., Newton, R. W., Greene, S. A., & Belch, J. J. (1999). Abnormal markers of endothelial cell activation and oxidative stress in children, adolescents and young adults with type 1 diabetes with no clinical vascular disease. Diabetes/Metabolism Research and Reviews, 15, 405–411.
Marra, G., Cotroneo, P., Pitocco, D., Manto, A., Di Leo, M. A., Ruotolo, V., et al. (2002). Early increase of oxidative stress and reduced antioxidant defenses in patients with uncomplicated type 1 diabetes: A case for gender difference. Diabetes Care, 25, 370–375.
Obrosova, I. G. (2009). Diabetic painful and insensate neuropathy: Pathogenesis and potential treatments. Neurotherapeutics, 6(4), 638–647.
Reynolds, T. M. (1963). Chemistry of nonenzymic browning. I. The reaction between aldoses and amines. Advances in Food Research, 12, 1–52.
Reynolds, T. M. (1965). Chemistry of nonenzymic browning. II. Advances in Food Research, 14, 167–283.
Kato, H., Hayase, F., Shin, D. B., Oimomi, M., & Baba, S. (1989). 3-Deoxyglucosone, an intermediate product of the Maillard reaction. Progress in Clinical and Biological Research, 304, 69–84.
Sell, D. R., & Monnier, V. M. (1989). Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. Journal of Biological Chemistry, 264(36), 21597–21602.
Sell, D. R., & Monnier, V. M. (1990). End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. The Journal of Clinical Investigation, 85(2), 380–384.
Grandhee, S. K., & Monnier, V. M. (1991). Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. Journal of Biological Chemistry, 266(18), 11649–11653.
Sell, D. R., Nagaraj, R. H., Grandhee, S. K., Odetti, P., Lapolla, A., Fogarty, J., & Monnier, V. M. (1991). Pentosidine: A molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes/Metabolism Reviews, 7(4), 239–251.
Dyer, D. G., Blackledge, J. A., Thorpe, S. R., & Baynes, J. W. (1991). Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. Journal of Biological Chemistry, 266(18), 11654–11660.
Miyata, S., & Monnier, V. (1992). Immunohistochemical detection of advanced glycosylation end products in diabetic tissues using monoclonal antibody to pyrraline. The Journal of Clinical Investigation, 89(4), 1102–1112.
Namiki, M., Hayashi, T., & Ohta, Y. (1977). Novel free radicals formed by the amino-carbonyl reactions of sugars with amino acids, amines, and proteins. Advances in Experimental Medicine and Biology, 86B, 471–501.
Hayashi, T., Ohta, Y., & Namiki, M. (1977). Electron spin resonance spectral study on the structure of the novel free radical products formed by the reactions of sugars with amino acids or amines. Journal of Agriculture and Food Chemistry, 25(6), 1282–1287.
Ahmed, M. U., Thorpe, S. R., & Baynes, J. W. (1986). Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. Journal of Biological Chemistry, 261(11), 4889–4894.
Baynes, J. W. (1991). Role of oxidative stress in development of complications in diabetes. Diabetes, 40(4), 405–412.
Dunn, J. A., Ahmed, M. U., Murtiashaw, M. H., Richardson, J. M., Walla, M. D., Thorpe, S. R., & Baynes, J. W. (1990). Reaction of ascorbate with lysine and protein under autoxidizing conditions: Formation of N epsilon-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochemistry, 29(49), 10964–10970.
Dunn, J. A., Patrick, J. S., Thorpe, S. R., & Baynes, J. W. (1989). Oxidation of glycated proteins: Age-dependent accumulation of N epsilon-(carboxymethyl)lysine in lens proteins. Biochemistry, 28(24), 9464–9468.
Dyer, D. G., Dunn, J. A., Thorpe, S. R., Bailie, K. E., Lyons, T. J., McCance, D. R., & Baynes, J. W. (1993). Accumulation of Maillard reaction products in skin collagen in diabetes and aging. The Journal of Clinical Investigation, 91(6), 2463–2469.
Thornalley, P., Wolff, S., Crabbe, J., & Stern, A. (1984). The autoxidation of glyceraldehyde and other simple monosaccharides under physiological conditions catalysed by buffer ions. Biochimica et Biophysica Acta, 797(2), 276–287.
Jiang, Z. Y., Woollard, A. C., & Wolff, S. P. (1990). Hydrogen peroxide production during experimental protein glycation. FEBS Letters, 268(1), 69–71.
Hunt, J. V., Dean, R. T., & Wolff, S. P. (1988). Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochemical Journal, 256(1), 205–212.
Hunt, J. V., Smith, C. C., & Wolff, S. P. (1990). Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes, 39(11), 1420–1424.
Wolff, S. P., & Dean, R. T. (1987). Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochemical Journal, 245, 243–250.
Babizhayev, M. A., Guiotto, A., & Kasus-Jacobi, A. (2009). N-Acetylcarnosine and histidyl-hydrazide are potent agents for multitargeted ophthalmic therapy of senile cataracts and diabetic ocular complications. Journal of Drug Targeting, 17(1), 36–63.
Thornalley, P. J., McLellan, A. C., Lo, T. W., Benn, J., & Sönksen, P. H. (1996). Negative association between erythrocyte reduced glutathione concentration and diabetic complications. Clinical Science (Lond)., 91(5), 575–582.
Honma, H., Podratz, J. L., & Windebank, A. J. (2003). Acute glucose deprivation leads to apoptosis in a cell model of acute diabetic neuropathy. Journal of the Peripheral Nervous System, 8, 65–74.
Ceriello, A., dello Russo, P., Amstad, P., & Cerutti, P. (1996). High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes, 45(4), 471–477.
Ceriello, A., Bortolotti, N., Falleti, E., Taboga, C., Tonutti, L., Crescentini, A., et al. (1997). Total radical-trapping antioxidant parameter in NIDDM patients. Diabetes Care, 20(2), 194–197.
Carolo dos Santos, K., Pereira Braga, C., Octavio Barbanera, P., Seiya, F. R., Fernandes Junior, A., & Fernandes, A. A. (2014). Cardiac energy metabolism and oxidative stress biomakers in diabetic rat treated with resveratrol. PLoS One, 9(7), e102775.
Maxwell, S. R., Thomason, H., Sandler, D., Leguen, C., Baxter, M. A., Thorpe, G. H., et al. (1997). Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. European Journal of Clinical Investigation, 27, 484–490.
Maxwell, S. R., Thomason, H., Sandler, D., Leguen, C., Baxter, M. A., Thorpe, G. H., et al. (1997). Poor glycaemic control is associated with reduced serum free radical scavenging (antioxidant) activity in non-insulin-dependent diabetes mellitus. Annals of Clinical Biochemistry, 34, 638–644.
van Dam, P. S., van Asbeck, B. S., Bravenboer, B., van Oirschot, J. F., Gispen, W. H., & Marx, J. J. (1998). Nerve function and oxidative stress in diabetic and vitamin E-deficient rats. Free Radical Biology and Medicine, 24(1), 18–26.
Chen, C. L., Liu, Q., & Relling, M. V. (1996). Simultaneous characterization of glutathione S-transferase M1 and T1 polymorphisms by polymerase chain reaction in American whites and blacks. Pharmacogenetics., 6(2), 187–191.
Iwata-Ichikawa, E., Kondo, Y., Miyazaki, I., Asanuma, M., & Ogawa, N. (1999). Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. Journal of Neurochemistry, 72(6), 2334–2344.
Ahmed, F. N., Naqvi, F. N., & Shafiq, F. (2006). Lipid peroxidation and serum antioxidant enzymes in patients with type 2 diabetes mellitus. Annals of the New York Academy of Sciences, 1084, 481–489.
Dickinson, P. J., Carrington, A. L., Frost, G. S., & Boulton, A. J. (2002). Neurovascular disease, antioxidants and glycation in diabetes. Diabetes/Metabolism Research and Reviews, 18(4), 260–272.
Seidegård, J., Vorachek, W. R., Pero, R. W., & Pearson, W. R. (1988). Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proceeding of the National Academy of Sciences of the USA, 85(19), 7293–7297.
Negi, G., Kumar, A., & Sharma, S. S. (2008). Oxidative stress in the pathophysiology of diabetic neuropathy: Mechanisms to management. Current Research & Information on Pharmaceutical Sciences, 9(4), 62–68.
Babizhayev, M. A., & Yegorov, Y. E. (2010). Therapeutic uses of drug-carrier systems for imidazole-containing dipeptide compounds that act as pharmacological chaperones and have significant impact on the treatment of chronic diseases associated with increased oxidative stress and the formation of advanced glycation end products. Critical Reviews in Therapeutic Drug Carrier Systems, 27(2), 85–154.
Babizhayev, M. A., & Yegorov, Y. E. (2010). Advanced drug delivery of N-acetylcarnosine (N-acetyl-beta-alanyl-l-histidine), carcinine (beta-alanylhistamine) and l-carnosine (beta-alanyl-l-histidine) in targeting peptide compounds as pharmacological chaperones for use in tissue engineering, human disease management and therapy: From in vitro to the clinic. Recent Patents on Drug Delivery & Formulation, 4(3), 198–230.
Acknowledgments
This work was planned, organized, and supported by Innovative Vision Products, Inc. (County of New Castle, DE, USA). Innovative Vision Products Inc. is a Pharmaceutical and Nanotechnology Development Company with a focus on innovative chemical entities, drug delivery systems, and unique medical devices to target specific biomedical applications. Over the last decade, IVP has developed a track record in developing these technologies to effectively address the unmet needs of specific diseased populations.
Conflict of interest
The authors report no conflict of interest in this work. The authors bear primary responsibility for accuracy of made statements and employment of the described products and for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babizhayev, M.A., Strokov, I.A., Nosikov, V.V. et al. The Role of Oxidative Stress in Diabetic Neuropathy: Generation of Free Radical Species in the Glycation Reaction and Gene Polymorphisms Encoding Antioxidant Enzymes to Genetic Susceptibility to Diabetic Neuropathy in Population of Type I Diabetic Patients. Cell Biochem Biophys 71, 1425–1443 (2015). https://doi.org/10.1007/s12013-014-0365-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0365-y