Abstract
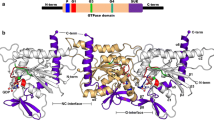
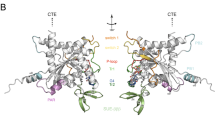
Septins form a conserved family of filament forming GTP binding proteins found in a wide range of eukaryotic cells. They share a common structural architecture consisting of an N-terminal domain, a central GTP binding domain and a C-terminal domain, which is often predicted to adopt a coiled-coil conformation, at least in part. The crystal structure of the human SEPT2/SEPT6/SEPT7 heterocomplex has revealed the importance of the GTP binding domain in filament formation, but surprisingly no electron density was observed for the C-terminal domains and their function remains obscure. The dearth of structural information concerning the C-terminal region has motivated the present study in which the putative C-terminal domains of human SEPT2, SEPT6 and SEPT7 were expressed in E. coli and purified to homogeneity. The thermal stability and secondary structure content of the domains were studied by circular dichroism spectroscopy, and homo- and hetero-interactions were investigated by size exclusion chromatography, chemical cross-linking, analytical ultracentrifugation and surface plasmon resonance. Our results show that SEPT6-C and SEPT7-C are able to form both homo- and heterodimers with a high α-helical content in solution. The heterodimer is elongated and considerably more stable than the homodimers, with a K D of 15.8 nM. On the other hand, the homodimer SEPT2-C has a much lower affinity, with a K D of 4 μM, and a moderate α-helical content. Our findings present the first direct experimental evidence toward better understanding the biophysical properties and coiled-coil pairings of such domains and their potential role in filament assembly and stability.







Similar content being viewed by others
Abbreviations
- SPR:
-
Surface plasmon resonance
- NTA:
-
Nitrilotriacetate
- IPTG:
-
Isopropyl-β-d-thiogalactopyranoside
- EGS:
-
Ethylene glycol-bis
- SDS/PAGE:
-
Sodium dodecylsulfate/polyacrylamide gel electrophoresis
- EDC:
-
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride
- NHS:
-
N-hydroxysuccinimide
- SEC:
-
Size exclusion chromatography
References
Hartwell, L. H. (1971). Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Experimental Cell Research, 69, 265–276.
Cao, L., Ding, X., Yu, W., Yang, X., Shen, S., & Yu, L. (2007). Phylogenetic and evolutionary analysis of the septin protein family in metazoan. FEBS Letters, 581, 5526–5532.
Pan, F., Malmberg, R. L., & Momany, M. (2007). Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evolutionary Biology, 7, 103.
Kartmann, B., & Roth, D. (2001). Novel roles for mammalian septins: From vesicle trafficking to oncogenesis. Journal of Cell Science, 114, 839–844.
Hsu, S. C., Hazuka, C. D., Roth, R., Foletti, D. L., Heuser, J., & Scheller, R. H. (1998). Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron, 20, 1111–1122.
Shcheprova, Z., Baldi, S., Frei, S. B., Gonnet, G., & Barral, Y. (2008). A mechanism for asymmetric segregation of age during yeast budding. Nature, 454, 728–734.
Spiliotis, E. T., Kinoshita, M., & Nelson, W. J. (2005). A mitotic septin scaffold required for Mammalian chromosome congression and segregation. Science, 307, 1781–1785.
Tooley, A. J., Gilden, J., Jacobelli, J., Beemiller, P., Trimble, W. S., Kinoshita, M., et al. (2009). Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nature Cell Biology, 11, 17–26.
Weirich, C. S., Erzberger, J. P., & Barral, Y. (2008). The septin family of GTPases: Architecture and dynamics. Nature Reviews Molecular Cell Biology, 9, 478–489.
Ihara, M., Tomimoto, H., Kitayama, H., Morioka, Y., Akigunchi, I., Shibasaki, H., et al. (2003). Association of the cytoskeletal GTP-binding protein Sept4/H5 with cytoplasmatic inclusions found in Parkinson’s disease and others synucleinopathies. The Journal of Biological Chemistry, 278, 24012–24095.
Hall, P. A., & Russell, S. E. (2004). The pathobiology of the septin gene family. The Journal of Pathology, 204, 489–505.
Mostowy, S., Nam Tham, T., Danckaert, A., Guadagnini, S., Boisson-Dupuis, S., Pizarro-Cerdá, J., et al. (2009). Septins regulate bacterial entry into host cells. PLoS One, 4, e4196.
Tanaka, M., Tanaka, T., Kijima, H., Itoh, J., Matsuda, T., Hori, S., et al. (2001). Characterization of tissue- and cell-type-specific expression of a novel human septin family gene, Bradeion. Biochemical and Biophysical Research Communications, 286, 547–553.
Garcia, W., de Araújo, A. P., Neto, Mde. O., Ballestero, M. R., Polikarpov, I., Tanaka, M., et al. (2006). Dissection of a human septin: definition and characterization of distinct domains within human SEPT4. Biochemistry, 45, 13918–13931.
Kinoshita, M. (2003). Assembly of mammalian septins. The Journal of Biochemistry, 134, 491–496.
Kinoshita, M. (2003). The septins. Genome Biology, 4, 236.
Field, C. M., al-Awar, O., Rosenblatt, J., Wong, M. L., Alberts, B., & Mitchison, T. J. (1996). A purified Drosophila septin complex forms filaments and exhibits GTPase activity. The Journal of Cell Biology, 133, 605–616.
Sirajuddin, M., Farkasovsky, M., Hauer, F., Kühlmann, D., Macara, I. G., Weyand, M., et al. (2007). Structural insight into filament formation by mammalian septins. Nature, 449, 311–315.
Sheffield, P. J., Oliver, C. J., Kremer, B. E., Sheng, S., Shao, Z., & Macara, I. G. (2003). Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. The Journal of Biological Chemistry, 278, 3483–3488.
Nagata, K., Asano, T., Nozawa, Y., & Inagaki, M. (2004). Biochemical and cell biological analyses of a mammalian septin complex, Sept7/9b/11. The Journal of Biological Chemistry, 279, 55895–55904.
Fujishima, K., Kiyonari, H., Kurisu, J., Hirano, T., & Kengaku, M. (2007). Targeted disruption of Sept3, a heteromeric assembly partner of Sept5 and Sept7 in axons, has no effect on developing CNS neurons. Journal of Neurochemistry, 102, 77–92.
Zhang, J., Kong, C., Xie, H., McPherson, P. S., Grinstein, S., & Trimble, W. S. (1999). Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Current Biology, 9, 1458–1467.
Hillebrand, S., Garcia, W., Delmar Cantú, M., de Araújo, A. P., Tanaka, M., Tanaka, T., et al. (2005). In vitro monitoring of GTPase activity and enzyme kinetics studies using capillary electrophoresis. Analytical and Bioanalytical Chemistry, 383, 92–97.
Nagaraj, S., Rajendran, A., Jackson, C. E., & Longtine, M. S. (2008). Role of nucleotide binding in septin–septin interactions and septin localization in Saccharomyces cerevisiae. Molecular and Cellular Biology, 28, 5120–5137.
Sirajuddin, M., Farkasovsky, M., Zent, E., & Wittinghofer, A. (2009). GTP-induced conformational changes in septins and implications for function. Proceedings of the National Academy of Sciences of the United States of America, 106, 16592–16597.
Lupas, A., Van Dyke, M., & Stock, J. (1991). Predicting coiled-coils from protein sequences. Science, 252, 1162–1164.
Wolf, E., Kim, P. S., & Berger, B. (1997). MultiCoil: A program for predicting two- and three-stranded coiled-coils. Protein Science, 6, 1179–1189.
Barth, P., Schoeffler, A., & Alber, T. (2008). Targeting metastable coiled-coil domains by computational design. Journal of the American Chemical Society, 130, 12038–12044.
Shinoda, T., Ito, H., Sudo, K., Iwamoto, I., Morishita, R., & Nagata, K. (2010). Septin 14 is involved in cortical neuronal migration via interaction with septin 4. Molecular Biology of the Cell, 21, 1324–1334.
Low, C., & Macara, I. G. (2006). Structural analysis of septin 2, 6, and 7 complexes. The Journal of Biological Chemistry, 281, 30697–30706.
Bertin, A., McMurray, M. A., Grob, P., Park, S. S., Garcia, G., 3rd, Patanwala, I., et al. (2008). Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proceedings of the National Academy of Sciences of the United States of America, 105, 8274–8279.
Sanger, F., Nicklen, S., & Coulson, R. (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 74, 5463–5467.
Gill, S. C., & von Hippel, P. H. (1989). Calculation of proteins extinction coefficients from amino acid sequence data. Analytical Biochemistry, 182, 319–326.
Sreerama, N., & Woody, R. W. (2000). Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Analytical Biochemistry, 287, 252–260.
Valadares, N. F., Polikarpov, I., & Garratt, R. C. (2008). Ligand induced interaction of thyroid hormone receptor beta with its coregulators. The Journal of Steroid Biochemistry and Molecular Biology, 112, 205–212.
Myszka, D. G. (1999). Improving biosensor analysis. Journal of Molecular Recognition, 12, 279–284.
Schuck, P., Perugini, M. A., Gonzales, N. R., Howlett, G. J., & Schubert, D. (2002). Size-distribution analysis of proteins by analytical ultracentrifugation: strategies and applications to model systems. Biophysical Journal, 82, 1096–1111.
Laue, T. M., Shah, B. D., Ridgeway, T. M., & Pelletier, S. L. (1992). Computer-aided interpretation of analytical sedimentation data for proteins. In S. E. Harding, H. C. Horton, & A. J. Rowe (Eds.), Analytical ultracentrifugation in biochemistry and polymer science (pp. 90–125). London, UK: Royal Society of Chemistry.
Cole, J. L. (2004). Analysis of heterogeneous interactions. Methods in Enzymology, 384, 212–232.
Cooper, T. M., & Woody, R. W. (1990). The effect of conformation on the CD of interacting helices: a theoretical study of tropomyosin. Biopolymers, 30, 657–676.
Rich, R. L., & Myszka, D. G. (2007). Survey of the year 2006 commercial biosensor literature. Journal of Molecular Recognition, 20, 300–366.
John, C. M., Hite, R. K., Weirich, C. S., Fitzgerald, D. J., Jawhari, H., Faty, M., et al. (2007). The Caenorhabditis elegans septin complex is nonpolar. The EMBO Journal, 26, 3296–3307.
Nakahira, M., Macedo, J. N. A., Seraphim, T. V., Cavalcante, N., Souza, T. A. C. B., Damalio, J. C. P., et al. (2010). A draft of the human septin interactome. PLoS One, 5, e13799.
Bertin, A., McMurray, M. A., Thai, L., Garcia, G., I. I. I., Votin, V., Grob, P., et al. (2010). Phosphatidylinositol-4, 5-bisphosphate promotes budding yeast septin filament assembly and organization. The Journal of Biological Chemistry, 404, 711–731.
Zhu, M., Wang, F., Yan, F., Yao, P. Y., Du, J., Gao, X., et al. (2008). Septin 7 interacts with centromere-associated protein E and is required for its kinetochore localization. The Journal of Biological Chemistry, 283, 18916–18925.
Acknowledgments
We acknowledge the financial support of FAPESP (via its CEPID program to the Centro de Biotecnologia Molecular Estrutural) and CNPq (via the INCT initiative). This work was further supported by a collaborative CSIC-CNPq grant for international exchange awarded to JMA and RCG as well as by grant MCINN BFU2008-00013 to JMA. IAM received a CAPES studentship and NFV, WG, JCPD and JNAM held bursaries from FAPESP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ivo de Almeida Marques and Napoleão Fonseca Valadares contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Almeida Marques, I., Valadares, N.F., Garcia, W. et al. Septin C-Terminal Domain Interactions: Implications for Filament Stability and Assembly. Cell Biochem Biophys 62, 317–328 (2012). https://doi.org/10.1007/s12013-011-9307-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-011-9307-0