Abstract
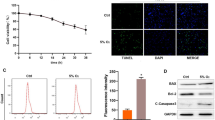
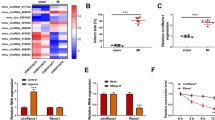
Cardiac Vascular disease particularly myocardial infarction (MI) is a threat to health worldwide. microRNAs (miRNAs) have been shown to regulate myocardial fibrosis. Therefore, it is potential to investigate the mechanism of miRNA and fibrosis following myocardial infarction. Hypoxia human cardiac microvascular endothelial cells (HCMECs) were selected for the vitro experimental model. The miR-146a-5p expression was tested via RT-qPCR. The level of endothelial-to-mesenchymal transition (EndMT) and fibrosis markers were detected by Western blotting and immunofluorescence. Then, the inflammation, cell viability and apoptosis were investigated. The target was predicted by an online database and verified by a dual-luciferase activity assay. An MI mouse model was created to validate that miR-146a-5p regulates cardiac fibrosis in vivo. MI mouse was transfected with miR-146a-5p lentivirus. Subsequently, its effect on cardiac fibrosis of infarcted hearts was assessed by In situ hybridization (ISH), Immunohistochemistry (IHC), Triphenylterazolium chloride (TTC) staining and Masson staining. Herein, we confirmed that miR-146a-5p was down-regulated in hypoxia HCMECs. Overexpression of miR-146a-5p inhibited hypoxia-induced cardiac fibrosis following myocardial infarction by inhibiting EndMT in HCMECs. Thioredoxin-interacting protein (TXNIP) was a target that was negatively regulated by miR-146a-5p. Up-regulation of miR-146a-5p inhibited cardiac fibrosis via regulating EndMT by targeting TXNIP, and it also regulated EndMT to inhibit cardiac fibrosis in vivo.






Similar content being viewed by others
Data Availability
Data sets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Roth, G. A., Mensah, G. A., & Fuster, V. (2020). The global burden of cardiovascular diseases and risks: A compass for global action. Journal of the American College of Cardiology, 76(25), 2980–2981.
Roth, G. A., Mensah, G. A., Johnson, C. O., Addolorato, G., Ammirati, E., Baddour, L. M., Barengo, N. C., Beaton, A. Z., Benjamin, E. J., Benziger, C. P., Bonny, A., Brauer, M., Brodmann, M., Cahill, T. J., Carapetis, J., Catapano, A. L., Chugh, S. S., Cooper, L. T., Coresh, J., … GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. Journal of the American College of Cardiology, 76(25), 2982–3021.
Adams, E., McCloy, R., Jordan, A., Falconer, K., & Dykes, I. M. (2021). Direct reprogramming of cardiac fibroblasts to repair the injured heart. Journal of Cardiovascular Development and Disease. https://doi.org/10.3390/jcdd8070072
Doenst, T., Haverich, A., Serruys, P., Bonow, R. O., Kappetein, P., Falk, V., Velazquez, E., Diegeler, A., & Sigusch, H. (2019). PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. Journal of the American College of Cardiology, 73(8), 964–976.
Francis Stuart, S. D., De Jesus, N. M., Lindsey, M. L., & Ripplinger, C. M. (2016). The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. Journal of Molecular and Cellular Cardiology, 91, 114–122.
Wu, Y., Zhang, S., Gong, X., Tam, S., Xiao, D., Liu, S., & Tao, Y. (2020). The epigenetic regulators and metabolic changes in ferroptosis-associated cancer progression. Molecular Cancer, 19(1), 39.
Yang, Y., Li, J., Rao, T., Fang, Z., & Zhang, J. (2021). The role and mechanism of hyperoside against myocardial infarction in mice by regulating autophagy via NLRP1 inflammation pathway. Journal of Ethnopharmacology, 276, 114187.
Li, J., Cai, S. X., He, Q., Zhang, H., Friedberg, D., Wang, F., & Redington, A. N. (2018). Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction. Basic Research in Cardiology, 113(5), 36.
Lionetti, V., Tuana, B. S., Casieri, V., Parikh, M., & Pierce, G. N. (2019). Importance of functional food compounds in cardioprotection through action on the epigenome. European Heart Journal, 40(7), 575–582.
Zhang, J., Lu, Y., Mao, Y., Yu, Y., Wu, T., Zhao, W., Zhu, Y., Zhao, P., & Zhang, F. (2022). IFN-γ enhances the efficacy of mesenchymal stromal cell-derived exosomes via miR-21 in myocardial infarction rats. Stem Cell Research & Therapy, 13(1), 333.
Huang, Y., Qi, Y., Du, J. Q., & Zhang, D. F. (2014). MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opinion on Therapeutic Targets, 18(12), 1355–1365.
Wang, X., Yang, C., Liu, X., & Yang, P. (2018). Ghrelin alleviates angiotensin II-induced H9c2 apoptosis: Impact of the miR-208 family. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research, 24, 6707–6716.
Lam, J., van den Bosch, M., Wegrzyn, J., Parker, J., Ibrahim, R., Slowski, K., Chang, L., Martinez-Høyer, S., Condorelli, G., Boldin, M., Deng, Y., Umlandt, P., Fuller, M., & Karsan, A. (2018). miR-143/145 differentially regulate hematopoietic stem and progenitor activity through suppression of canonical TGFβ signaling. Nature Communications, 9(1), 2418.
Zhang, L., He, J., Wang, J., Liu, J., Chen, Z., Deng, B., Wei, L., Wu, H., Liang, B., Li, H., Huang, Y., Lu, L., Yang, Z., Xian, S., & Wang, L. (2021). Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy. Cell Death & Disease, 12(5), 470.
Mao, J., Liu, J., Zhou, M., Wang, G., Xiong, X., & Deng, Y. (2022). Hypoxia-induced interstitial transformation of microvascular endothelial cells by mediating HIF-1α/VEGF signaling in systemic sclerosis. PLoS ONE, 17(3), e0263369.
Iwano, M., Plieth, D., Danoff, T. M., Xue, C., Okada, H., & Neilson, E. G. (2002). Evidence that fibroblasts derive from epithelium during tissue fibrosis. The Journal of Clinical Investigation, 110(3), 341–350.
Brokopp, C. E., Schoenauer, R., Richards, P., Bauer, S., Lohmann, C., Emmert, M. Y., Weber, B., Winnik, S., Aikawa, E., Graves, K., Genoni, M., Vogt, P., Lüscher, T. F., Renner, C., & Hoerstrup, S. P. (2011). Matter CM Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. European Heart Journal, 32(21), 2713–2722.
Jensen, E. C. (2013). Quantitative analysis of histological staining and fluorescence using ImageJ. Anatomical Record (Hoboken, NJ : 2007), 296(3), 378–381.
Iourov, I. Y. (2017). Quantitative fluorescence in situ hybridization (QFISH). Methods in Molecular Biology (Clifton, NJ), 1541, 143–149.
Varghese, F., Bukhari, A. B., Malhotra, R., & De, A. (2014). IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE, 9(5), e96801.
Ruifrok, A. C., & Johnston, D. A. (2001). Quantification of histochemical staining by color deconvolution. Analytical and Quantitative Cytology and Histology, 23(4), 291–299.
Leask, A. (2015). Getting to the heart of the matter: New insights into cardiac fibrosis. Circulation Research, 116(7), 1269–1276.
Schelbert, E. B., Fonarow, G. C., Bonow, R. O., Butler, J., & Gheorghiade, M. (2014). Therapeutic targets in heart failure: Refocusing on the myocardial interstitium. Journal of the American College of Cardiology, 63(21), 2188–2198.
Cheng, R., Dang, R., Zhou, Y., Ding, M., & Hua, H. (2017). MicroRNA-98 inhibits TGF-β1-induced differentiation and collagen production of cardiac fibroblasts by targeting TGFBR1. Human Cell, 30(3), 192–200.
van Rooij, E., Sutherland, L. B., Thatcher, J. E., DiMaio, J. M., Naseem, R. H., Marshall, W. S., Hill, J. A., & Olson, E. N. (2008). Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences of the United States of America, 105(35), 13027–13032.
Guan, H., Shang, G., Cui, Y., Liu, J., Sun, X., Cao, W., Wang, Y., & Li, Y. (2019). Long noncoding RNA APTR contributes to osteosarcoma progression through repression of miR-132-3p and upregulation of yes-associated protein 1. Journal of Cellular Physiology, 234(6), 8998–9007.
Gao, X. F., Wang, Z. M., Wang, F., Gu, Y., Zhang, J. J., & Chen, S. L. (2019). Exosomes in coronary artery disease. International Journal of Biological Sciences, 15(11), 2461–2470.
Fraccarollo, D., Galuppo, P., & Bauersachs, J. (2012). Novel therapeutic approaches to post-infarction remodelling. Cardiovascular Research, 94(2), 293–303.
Viola, M., de Jager, S. C. A., & Sluijter, J. P. G. (2021). Targeting inflammation after myocardial infarction: A therapeutic opportunity for extracellular vesicles? International Journal of Molecular Sciences, 22(15), 7831.
Frangogiannis, N. G. (2012). Regulation of the inflammatory response in cardiac repair. Circulation Research, 110(1), 159–173.
Ueland, T., Gullestad, L., Nymo, S. H., Yndestad, A., Aukrust, P., & Askevold, E. T. (2015). Inflammatory cytokines as biomarkers in heart failure. Clinica Chimica Acta: International Journal of Clinical Chemistry, 443, 71–77.
Panahi, M., Papanikolaou, A., Torabi, A., Zhang, J. G., Khan, H., Vazir, A., Hasham, M. G., Cleland, J. G. F., Rosenthal, N. A., Harding, S. E., & Sattler, S. (2018). Immunomodulatory interventions in myocardial infarction and heart failure: A systematic review of clinical trials and meta-analysis of IL-1 inhibition. Cardiovascular Research, 114(11), 1445–1461.
Hu, J., & Yu, Y. (2018). The function of thioredoxin-binding protein-2 (TBP-2) in different diseases. Oxidative Medicine and Cellular Longevity, 2018, 4582130.
Domingues, A., & Jolibois, J. (2021). Marquet de Rougé P, Nivet-Antoine V: The emerging role of TXNIP in ischemic and cardiovascular diseases; A novel marker and therapeutic target. Int J Mol Sci, 22(4), 1693.
Narasimhan, B., Narasimhan, H., Lorente-Ros, M., Romeo, F. J., Bhatia, K., & Aronow, W. S. (2021). Therapeutic angiogenesis in coronary artery disease: A review of mechanisms and current approaches. Expert Opinion on Investigational Drugs, 30(9), 947–963.
Acknowledgements
We appreciate our colleagues for their helpful suggestions.
Funding
This study was supported by The National Natural Science Foundation of China (No. 82160076), Young Talents of Yunnan Thousand Talents Plan (No. RLQB20220011), Yunnan Provincial Department of Science and Technology (No. 202301AT070093), Special Foundation Projects of Joint Applied Basic Research of Yunnan Provincial Department of Science and Technology with Kunming Medical University (No. 202201AY070001-062), 535 Talent Project of First Affiliated Hospital of Kunming Medical University (No. 2023535Q04), Hospital management project of 920th Hospital of Joint Logistics Support Force of Chinese People's Liberation Army (2019YGC06), The Reserve Talents’ Project of Science and Technology Pioneer in Yunnan Province. (No. 2019HB045).
Author information
Authors and Affiliations
Contributions
YW and JY: were responsible for the conceptualization of the project. YZ and CO: were responsible for the experimental design, application and manuscript writing. LC, WC, WW, SH, JH, GS, and LL: provided valuable suggestions for the manuscript and experiments. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no competing interests to declare in relation to the content of this article.
Ethical Approval
Yunnan Labreal Co., Ltd. assisted with the animal experiments and was approved by the Ethics Committee. (Issue No. PZ20211003).
Consent for Publication
Upon request, the corresponding author can provide some or all of the data, models, or codes generated or used during the study.
Additional information
Handling Editor: Daniel Conklin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12012_2023_9818_MOESM1_ESM.tif
Supplementary file1 (TIF 43233 KB) The immunofluorescence of α-SMA and Ve-cadherin in mouse heart section, x20, scale bar: 50um, n=6.
12012_2023_9818_MOESM2_ESM.tif
Supplementary file2 (TIF 45826 KB) The immunofluorescence of α-SMA and Ve-cadherin in mouse heart section, x20, scale bar: 50um, n=6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Yu, J., Ou, C. et al. miRNA-146a-5p Inhibits Hypoxia-Induced Myocardial Fibrosis Through EndMT. Cardiovasc Toxicol 24, 133–145 (2024). https://doi.org/10.1007/s12012-023-09818-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-023-09818-1