Abstract
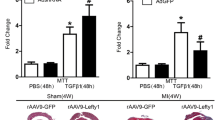
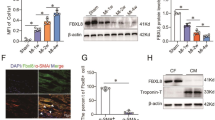
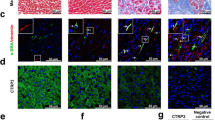
Notch3 and TGF-β1 signaling play a key role in the pathogenesis and progression of chronic cardiovascular disease. However, whether Notch3 protects against myocardial infarction (MI) and the underlying mechanisms remains unknown. C57BL/6 mice were randomized to be treated with Notch3 siRNA (siNotch3) or lentivirus carrying Notch3 cDNA (Notch3) before coronary artery ligation. Four weeks after constructing MI model, cardiac function and fibrosis were compared between groups. The cardiac fibroblast cells (CFs) were isolated from newborn C57BL/6 mice (1–3 days old) and transfected with lentivirus carrying Notch3 cDNA. TGF-β1 (5 ng/ml), a well-known pro-fibrotic factor, was administered 72 h after Notch3 cDNA administration in CFs. The related proteins of fibrosis such as a-smooth muscle actin (a-SMA), Type I collagen, metalloprotease (MMP)-9 and the tissue inhibitor of metalloproteinases (TIMP)-2 were examined by western blot analysis. Notch3 cDNA treatment attenuated cardiac damage and inhibited fibrosis in mice with MI. Meanwhile, Notch3 siRNA administration aggravated cardiac function damage and markedly enhanced cardiac fibrosis in mice with MI. Overexpression of Notch3 inhibited TGF-β1-induced fibroblast–myofibroblast transition of mouse cardiac fibroblast cells, as evidenced by down-regulating a-SMA and Type I collagen expression. Notch3 cDNA treatment also increased MMP-9 expression and decreased TIMP-2 expression in the TGF-β1-stimulated cells. This study indicates that Notch3 is an important protective factor for cardiac fibrosis in a MI model, and the protective effect of Notch3 is attributable to its action on TGF-β1/Smad3 signaling.




Similar content being viewed by others
References
Leask, A. (2010). Potential therapeutic targets for cardiac fibrosis: TGF beta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circulation Research, 106, 1675–1680.
Flanders, K. C. (2004). Smad3 as a mediator of the fibrotic response. International Journal of Experimental Pathology, 85, 47–64.
Sassoli, C., Chellini, F., Pini, A., Tani, A., Nistri, S., Nosi, D., et al. (2013). Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-b/Smad3 signaling. PLoS ONE, 8, e63896.
Docherty, N. G., Murphy, M., Martin, F., Brennan, E. P., & Godson, C. (2014). Targeting cellular drivers and counter-regulators of hyperglycaemia and TGF-β1 associated pro-fibrotic responses in diabetic kidney disease. Experimental Physiology, 99, 1154–1162.
Vivar, R., Humeres, C., Ayala, P., Olmedo, I., Catalán, M., García, L., et al. (2013). TGF-β1 prevents simulated ischemia/reperfusion-induced cardiac fibroblast apoptosis by activation of both canonical and non-canonical signaling pathways. Biochimica et Biophysica Acta, 1832, 754–762.
Lassaletta, A. D., Elmadhun, N. Y., Burgess, T. A., Bianchi, C., Sabe, A. A., Robich, M. P., et al. (2014). Microvascular Notch signaling is upregulated in response to vascular endothelial growth factor and chronic myocardial ischemia. Circulation Journal, 78, 743–751.
Zhou, X. L., & Liu, J. C. (2014). Role of Notch signaling in the mammalian heart. Brazilian Journal of Medical and Biological Research, 47, 1–10.
Artavanis-Tsakonas, S., Rand, M. D., & Lake, R. J. (1999). Notch signaling: Cell fate control and signal integration in development. Science, 284, 770–776.
Bray, S. J. (2006). Notch signalling: A simple pathway becomes complex. Nature Reviews Molecular Cell Biology, 7, 678–689.
Mazzone, M., Selfors, L. M., Albeck, J., Overholtzer, M., Sale, S., Carroll, D. L., et al. (2010). Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proceedings of the National Academy of Sciences of the United States of America, 107, 5012–5017.
Wickremasinghe, R. G., Prentice, A. G., & Steele, A. J. (2011). p53 and Notch signaling in chronic lymphocytic leukemia: Clues to identifying novel therapeutic strategies. Leukemia, 25, 1400–1407.
Kennard, S., Liu, H., & Lilly, B. (2008). Transforming growth factor-beta (TGF-1) down-regulates Notch-3 in fibroblasts to promote smooth muscle gene expression. Journal of Biological Chemistry, 283, 1324–1333.
MacGrogan, D., Nus, M., & de la Pompa, J. L. (2010). Notch signaling in cardiac development and disease. Current Topics in Developmental Biology, 92, 333–365.
Hurlbut, G. D., Kankel, M. W., Lake, R. J., & Artavanis-Tsakonas, S. (2007). Crossing paths with Notch in the hyper-network. Current Opinion in Cell Biology, 19, 166–175.
Fan, Y. H., Dong, H., Pan, Q., Cao, Y. J., Li, H., & Wang, H. C. (2011). Notch signaling may negatively regulate neonatal rat cardiac fibroblast-myofibroblast transformation. Physiological Research, 60, 739–748.
Gude, N. A., Emmanuel, G., Wu, W., Cottage, C. T., Fischer, K., Quijada, P., et al. (2008). Activation of Notch-mediated protective signaling in the myocardium. Circulation Research, 102, 1025–1035.
Gao, E., Lei, Y. H., Shang, X., Huang, Z. M., Zuo, L., Boucher, M., et al. (2010). A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circulation Research, 107, 1445–1453.
Li, P., Wang, D., Lucas, J., Oparil, S., Xing, D., Cao, X., et al. (2008). A trial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circulation Research, 102, 185–192.
Li, Y., Hiroi, Y., Ngoy, S., Okamoto, R., Noma, K., Wang, C. Y., et al. (2011). Notch1 in bone marrow derived cells mediates cardiac repair after myocardial infarction. Circulation, 123, 866–876.
Zakharova, L., Nural-Guvener, H., Nimlos, J., Popovic, S., & Gaballa, M. A. (2013). Myocardial infarction is associated with transforming growth factor beta-dependent yield and functional decline in atrial explant-derived c-Kit + cells. Journal of the American Heart Association, 2, e000317.
Kratsios, P., Catela, C., Salimova, E., Huth, M., Berno, V., Rosenthal, N., et al. (2010). Distinct roles for cell-autonomous notch signaling in cardiomyocytes of the embryonic and adult heart. Circulation Research, 106, 559–572.
Ge, W., & Ren, J. (2012). mTOR-STAT3-notch signalling contributes to ALDH2-induced protection against cardiac contractile dysfunction and autophagy under alcoholism. Journal of Cellular and Molecular Medicine, 16, 616–626.
Russell, J. L., Goetsch, S. C., Gaiano, N. R., Hill, J. A., Olson, E. N., & Schneider, J. W. (2011). A dynamic Notch injury response activates epicardium and contributes to fibrosis-repair. Circulation Research, 108, 51–59.
Li, Y., Hiroi, Y., & Liao, J. K. (2010). Notch signaling as an important mediator of cardiac repair and regeneration after myocardial infarction. Trends in Cardiovascular Medicine, 20, 228–231.
Visse, R., & Nagase, H. (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circulation Research, 92, 827–839.
Barnes, B. R., Szelenyi, E. R., Warren, G. L., & Urso, M. L. (2009). Alterations in mRNA and protein levels of metalloproteinases-2, -9, and -14 and tissue inhibitor of metalloproteinase-2 responses to traumatic skeletal muscle injury. American Journal of Physiology. Cell Physiology, 297, C1501–C1508.
Lu, K. V., Jong, K. A., Rajasekaran, A. K., Cloughesy, T. F., & Mischel, P. S. (2004). Upregulation of tissue inhibitor of metalloproteinases (TIMP)-2 promotes matrix metalloproteinase (MMP)-2 activation and cell invasion in a human glioblastoma cell line. Laboratory Investigation, 84, 8–20.
Yang, J., Zheng, J., Wu, L., Shi, M., Zhang, H., Wang, X., et al. (2011). NDRG2 ameliorates hepatic fibrosis by inhibiting the TGF-b1/Smad pathway and Altering the MMP2/TIMP2 ratio in rats. PLoS ONE, 6, e27710.
Huang, X. Z., Wen, D., Zhang, M., Xie, Q., Ma, L., Guan, Y., et al. (2014). Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-b/Smad3 pathway. Journal of Cellular Biochemistry, 115, 996–1005.
Feng, X. H., & Derynck, R. (2005). Specificity and versatility in TGF-beta signaling through Smads. Annual Review of Cell and Developmental Biology, 21, 659–693.
Samarakoon, R., Overstreet, J. M., & Higgins, P. J. (2013). TGF-b signaling in tissue fibrosis: Redox controls, target genes and therapeutic opportunities. Cellular Signalling, 25, 264–268.
Nemir, M., Metrich, M., Plaisance, I., Lepore, M., Cruchet, S., Berthonneche, C., et al. (2014). The Notch pathway controls fibrotic and regenerative repair in the adult heart. European Heart Journal, 35, 2174–2185.
Acknowledgments
This work was supported by National Nature Science Foundation of China (Nos. 81570318, 81300149, 81270263) and Shaanxi Social Development and Scientific Problem Tackling Program (2015SF097).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhang, M., Pan, X., Zou, Q. et al. Notch3 Ameliorates Cardiac Fibrosis After Myocardial Infarction by Inhibiting the TGF-β1/Smad3 Pathway. Cardiovasc Toxicol 16, 316–324 (2016). https://doi.org/10.1007/s12012-015-9341-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-015-9341-z