Abstract
Lactoferrin is a natural cationic iron-binding glycoprotein of the transferrin family found in bovine milk and other exocrine secretions, including lacrimal fluid, saliva, and bile. Lactoferrin has been investigated for its numerous powerful influences, including anticancer, anti-inflammatory, anti-oxidant, anti-osteoporotic, antifungal, antibacterial, antiviral, immunomodulatory, hepatoprotective, and other beneficial health effects. Lactoferrin demonstrated several nutraceutical and pharmaceutical potentials and have a significant impact on improving the health of humans and animals. Lactoferrin plays a critical role in keeping the normal physiological homeostasis associated with the development of pathological disorders. The current review highlights the medicinal value, nutraceutical role, therapeutic application, and outstanding favorable health sides of lactoferrin, which would benefit from more exploration of this glycoprotein for the design of effective medicines, drugs, and pharmaceuticals for safeguarding different health issues in animals and humans.
Similar content being viewed by others
Introduction
The health of animals and humans is disturbed by several microbial agents, and disease is a key constraint on the productive performance of animals. Several decades ago, antimicrobials were widely used for an extended period to prevent and treat infectious disorders in animals and humans. With the emergence of side effects of chemosynthetic medicine and antibiotic resistance, there has been a surge in interest among researchers, the general public, and the scientific community worldwide in the search for alternative sources of therapeutic agents [1,2,3]. It has been reported that several biological compounds found in medicinal plants and their derivatives have been extensively studied for their potential health benefits and therapeutic properties. These compounds can be used in various forms, such as extracts, essential oils, and phytochemicals, to treat a wide range of ailments and health conditions in both humans and animals [4,5,6,7,8,9]. Furthermore, with the development of technology and science, numerous medicinal compounds, including phytoconstituents, herbs, phytochemicals, and animal protein, have been scientifically validated, promoted, popularized, and applied in livestock and humans to treat pathological conditions [10,11,12,13]. It can be associated with their well-recognized and favorable health-promoting applications and therapeutic potential against both infectious and non-infectious disorders in humans and livestock [14,15,16,17].
Lactoferrin is a non-toxic cationic glycosylated globular protein, first identified as the red protein of mammalian milk. Later on, it has been explained as an iron-binding protein owing to its sequestration of Fe3+ and Fe2+ free ions and thus is included in the metalloproteins group [18, 19]. The molecular weight of lactoferrin is 78 to 80 kDa containing approximately 690 amino acids (AA) and is found in several exocrine secretions of mammals such as colostrum, milk, uterine secretions, saliva, tears, and seminal fluid [20]. Human and animal colostrum contains higher concentrations of lactoferrin than whole milk. It was reported that the concentration of lactoferrin in bovine colostrum is about 2–5 mg/ml, whereas the concentration of lactoferrin in whole milk is about 0.1–0.3 mg/ml, which is much lower than human milk (2–3 g/L) [21,22,23]. Furthermore, secondary granules of neutrophils also produce lactoferrin against infection and induce immunological responses [24]. Lactoferrin is a safe and potent nutraceutical and pharmaceutical protein alternative to chemotherapeutic agents and is readily available in the commercial market. Thus, lactoferrin could be used as a potential curative agent in veterinary medicine and human clinics as an alternative or substitute to synthetic antibiotics to treat infectious disorders [25, 26]. Lactoferrin can be used as a dietary supplement as a functional food or nutraceutical.
Lactoferrin showed potential multidimensional and holistic favorable influences, including anti-inflammatory, immunomodulatory, antioxidant, antibacterial, antiviral, neuroprotective, bone repairing, and safeguarding numerous health disorders such as cancerous conditions and lifestyle illnesses like obesity, digestive problems, high blood pressure, diabetes, hyperlipidemia, and others [27,28,29]. Lactoferrin has been displayed to be beneficial against emerging pathogens such as coronavirus and other naked and enveloped RNA and DNA viruses like human immunodeficiency virus (HIV), herpes simplex virus, and human papillomavirus [30, 31]. Lactoferrin underlying antimicrobial mechanism of action against a diverse array of microorganisms is attributed to the iron-binding or iron-chelating ability of this protein, besides its molecular or cellular interaction with both infectious pathogens and the host [32,33,34]. This review aims to provide current knowledge regarding the nutraceutical, pharmacological, pharmaceutical, therapeutic, and remedial properties of bovine lactoferrin for treating numerous pathological and chronic diseases in animals and humans.
History and Chemical Structure of Lactoferrin
Lactoferrin is a multifunctional iron-binding glycoprotein that was first discovered in 1939 by Sorensen and colleagues while studying the antimicrobial properties of bovine milk [35]. It is found in various mammalian secretions, including colostrum, milk, saliva, tears, and mucous, where it plays a crucial role in the innate immune defense system. Since its discovery, lactoferrin has been the subject of extensive research, and its biological functions have been widely investigated. The protein has been shown to exhibit various biological activities, including antimicrobial, antiviral, antitumor, anti-inflammatory, and immunomodulatory properties [36]. However, it was not until the 1960s that lactoferrin was purified and characterized as an iron-binding protein.
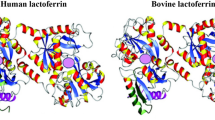
The first crystal structure of lactoferrin was determined in 1984 by Anderson and colleagues [36]. This landmark study provided insight into the structural features of the protein and helped to explain its diverse biological activities. The chemical structure of lactoferrin is composed of a single polypeptide chain of approximately 700 amino acids, with a molecular weight of approximately 80 kDa. It is folded into two lobes, each of which contains an iron-binding site. The N-terminal lobe has a higher affinity for iron than the C-terminal lobe [37, 38]. Since then, numerous studies have been conducted to elucidate the functional properties of lactoferrin and its potential therapeutic applications. The chemical structure of lactoferrin is displayed in Fig. 1.
Crystal structure of bovine lactoferrin (PBD code = 1BLF), human lactoferrin (1B0L). Adopted from Vogel (2012) [24]. Pink spheres represents the ferric ion (Fe+3) binding sites
Sources of Lactoferrin
Lactoferrin is a key constituent of innate immune function that leads to the activation of the adaptive immune system. It is constantly produced and released into the exocrine secretion, including tears, saliva, vaginal, and seminal fluid. However, milk is a significant source of lactoferrin, secreted from the mammary glands via glandular epithelial cells. Table 1 shows the sources and concentration of lactoferrin in different types of milk and colostrum. The amount of lactoferrin in milk varies depending on the several factors, including the lactation stage, health status, and animal species. Lactoferrin is present in high concentrations in human colostrum, with levels ranging from 5 to 6 g per liter; however, the mature milk and secretion contains much lower level depending on the stage of lactation and other factors. The concentration of lactoferrin in human colostrum can range from 1 to 2 g per liter, which is significantly higher than the levels found in mature milk [39]. Another study found that the concentration of lactoferrin in human milk varied between Chinese populations during lactation. The colostrum samples had the highest concentration of lactoferrin (3.16 g/L), followed by transitional milk and mature milk (1.73 g/L and 0.90 g/L, respectively) [40]. Despite the nutraceutical role of lactoferrin, it has been demonstrated to have multiple health-promoting and pharmaceutical potentials. For these reasons, lactoferrin was isolated from the milk of numerous animal species, including camels, bovines, goats, and humans (Fig. 2). The potential biological impacts of milk-derived lactoferrin are summarized in Table 2.
Nutraceutical Role of Lactoferrin
Recently, nutraceuticals, including carbohydrates, proteins, amino acids, vitamins, and minerals, have gained more attention due to various health and nutritional benefits, for instance, preventing disease, maintaining normal physiological status, and thus boosting the productive performance and health status of animals and humans [69,70,71,72,73]. Lactoferrin is known as the red fraction of milk, is a non-heme iron-binding glycoprotein, and is newly considered a nutraceutical protein [74]. Lactoferrin act as an important component of the innate immune system and has a wide range of biological functions [75]. Previously, it was reported that lactoferrin supplementation led to changes in the composition and diversity of the gut microbiota, including an increase in beneficial bacteria such as Bifidobacterium and Lactobacillus, and a decrease in potentially harmful bacteria such as Enterobacteriaceae [76]. Another study found that lactoferrin can enhance both innate and adaptive immune responses, including the activation of immune cells such as macrophages, dendritic cells, and T cells [77]. According to Konstanti et al. [76], bovine lactoferrin supplementation to healthy elderly women increased Holdemanella’s relative abundance. Moreover, adding active galactooligosaccharides (GOS), 2.64 g/day simultaneously for a further 3 weeks, increased the relative abundance of Bifidobacterium in the fecal microbiota. Aside from that, the assessed gut indicators and the prebiotic activity of GOS were unaffected by adding bovine lactoferrin supplementation, which was not harmful to intestinal health.
For instance, bone fragility is a significant issue for laying hens kept in cages; therefore, lactoferrin, one of the bone anabolic components, was successful in increasing the strength and weight of tibia bones as well as the weight of eggs in laying hens by injecting lactoferrin into their eggs at a rate of 67.5 g per egg [78]. In the Humphrey et al. [79] study, chicks were given rice that had been genetically modified to express human lysozyme and lactoferrin with a total amount of transgenic plus conventional rice of 20% in corn soya-based ration to evaluate the impact on growth performance and intestinal health. The chicks fed 5% lactoferrin + 10% lysozyme + 5% conventional rice ration outperformed those fed the 20% conventional rice ration in terms of lamina propria thickness in the duodenum and feed efficiency.
Lactoferrin is a potent antimicrobial constituent against a wide range of pathogens and contributes to specific immune responses [38, 75]. The bioavailability of lactoferrin would be a significant concern when used as a nutraceutical supplement for treating infectious diseases. It has been suggested that the external coating of lactoferrin improves the uptake of lactoferrin in the small intestine [80]. Enteric coating protects the enzymatic degradation of lactoferrin in the stomach and allows it to bind to small intestine lactoferrin receptors for absorption and transit into the systemic circulation [80]. Another study found that enteric-coated lactoferrin had significantly higher bioavailability and absorption (approximately ten times higher) than non-coated lactoferrin [81]. Given the previous literature, we assumed that enteric-coated lactoferrin has superior biological properties than regular lactoferrin concerning bioavailability, leading to therapeutic potential against infectious disorders.
Recombinant Human Lactoferrin, Production, and Application
Recombinant human lactoferrin (rhLF) is a protein that is produced using genetic engineering techniques. It has been found to have several nutraceutical roles, which means it has potential health benefits beyond basic nutrition [82]. Recombinant human lactoferrin is produced by introducing the gene for lactoferrin into a host cell, such as a bacterial or mammalian cell, which then produces the lactoferrin protein. This protein can be purified and used for various purposes, such as in medical research or as a supplement. Table 3 summarizes the various attempts made to produce recombinant human lactoferrin using different expression systems. Among the various expression systems, only a few have been successfully scaled up at industrial level. These include rhLF from Aspergillus awamori, which is produced by Agennix (Houston, TX, USA); rhLF from rice, which is produced by Ventria Bioscience (Sacramento, CA, USA); and rhLF from transgenic cows, which is produced by Pharming (Leiden, The Netherlands). These successful production methods have the potential to provide a sustainable source of rhLF for various applications, such as in infant formula, dietary supplements, and medical treatments [83].
In recent years, there has been growing interest in the use of transgenic animals, fungi, higher plants, and cell cultures for the production of recombinant human lactoferrin [83]. Published studies have suggested that rhLF derived from transgenic cow milk is quite similar to the natural human lactoferrin in concentration (1.5–3.4 g/L) and in biological properties [106]; however, other transgenic animals showed similar or even higher expression [107]. It was reported that mammalian expression systems have potential disadvantages, such as viral contamination of target proteins and bioethical concerns, which may lead some researchers to explore alternative expression systems such as yeast, filamentous fungi, and higher plants. Interestingly, it has been found that rice could be the promising plant system for the synthesis of rhLF owing to the higher production level (5.0 g/kg). Additionally, rice plant system is more appealing option for the production of safe and hypoallergic rhLF compared to other plants or microbial systems [108]. However, researchers continuously explore different expression systems to find the most efficient and effective way to produce important proteins like rhLF. Recent study has been reported that cell culture–derived rhLF produced through cell culture techniques may be introduced as bio-similar compound in the near future [83]. However, the commercial production bio-similar compound of rhLF has not yet been started.
Recombinant human lactoferrin is a protein that has been extensively studied for its potential therapeutic effects in various pathophysiological and conditions, and shown similar biological activities to native lactoferrin, making it a promising alternative for pharmaceutical and nutraceutical applications. In the pharmaceutical industry, rhLF has been investigated as a potent therapeutic compound for the treatment of various diseases [74]. Research has shown that this protein effectively inhibits the growth of Escherichia coli and herpes simplex virus, among other pathogens. In addition to its antimicrobial properties, this compound also used to correct the abnormal inflammatory conditions such as arthritis and colitis [109]. The rhLF also promoted the growth of gut beneficial bacteria leading to enhance the absorption of available iron. It may also have potential benefits for athletes, as it has been shown to reduce oxidative stress and improve exercise performance. In conclusion, the rhLF is a potent iron bearing protein with superior functional and pharmaceutical properties [110]. The multiple health benefits of this compound make it a promising candidate for future research.
Therapeutic Potential of Lactoferrin
In recent years, lactoferrin has gained much attention towards research community owing to the potential therapeutic properties against numbers of pathophysiological disorders. The important biological functions associated with lactoferrin supplementation are illustrated in Fig. 2. Guo et al. [111] reported that dietary lactoferrin improves bone mass, bone strength, and bone mineral density, leading to the restoration of the microarchitecture of bone in ovariectomized rats. Additionally, another study showed that formulated lactoferrin (milk ribonuclease-enriched lactoferrin) improved bone mineralization and bone turnover in 38 healthy postmenopausal women [112]. It has been demonstrated that lactoferrin supplementation minimizes the incidence of osteoporosis, but more investigations are required to confirm these effects [113]. Maternal iron binding lactoferrin supplementation improved human fetal growth [114, 115].
Lactoferrin Is a Key Ingredient for Infant Formula
Lactoferrin is an important ingredient in some infant formulas. In breast milk, lactoferrin is present in relatively high concentrations and has been shown to provide various health benefits to infants, including improved gut health and immunity [116]. Infant formula manufacturers have therefore sought to include lactoferrin in their products to try and replicate some of these benefits [117]. However, the levels of lactoferrin in most infant formulas are much lower than those in breast milk, and it is not clear to what extent lactoferrin supplementation in formula can provide the same benefits as natural lactoferrin in breast milk. King Jr et al. [118] suggested that bovine lactoferrin supplementation at the level of 850 mg/l could be safe and well-tolerated in bottle-fed infants. However, there was non-significant difference in the incidence of gastrointestinal and respiratory tract infections between the lactoferrin and placebo groups. Moreover, non-significant effect was also observed on the growth parameters between the two groups. A double-blind, randomized, controlled trial, investigated the growth and tolerance of infants fed formula containing bovine lactoferrin (0.6 or 1.0 g/L); the infants were monitored for 1 year, and their growth and tolerance of the formulas were assessed [119]. Results showed non-significant differences in growth between the infants who were fed the standard formula and those who were fed the lactoferrin-enriched formula. However, the infants who were fed the lactoferrin-enriched formula had fewer episodes of diarrhea and respiratory infections compared to those who were fed the standard formula [119]. Li et al. [117] investigated the biological effect of adding bovine milk fat globule membrane and lactoferrin (0.6 g/L) to infant formula in infants. It was found that infants who received the experimental formula had significantly better cognitive, language, and motor development scores at 12 months of age compared to those who received the control formula. Chen et al. [120] investigated the effect of graded level of bovine lactoferrin on the iron metabolism of anemic infants. The results indicated that both lactoferrin-fortified groups had significantly higher levels of serum iron, ferritin, and transferrin saturation compared to the control group at the 12th week. However, there was non-significant difference between the low-dose and high-dose groups, suggesting that 25 mg/L of lactoferrin was sufficient to improve iron metabolism in anemic infants. Similarly, another study demonstrated that the supplementation of lactoferrin and/or galacto-oligosaccharides to the infant formulas may have improved the bio-accessibility of iron by enhancing its solubility and availability for absorption [121].
Currently, lactoferrin has been added to infant formula due to its potential benefits for infants. The European Food Safety Authority (EFSA) is a scientific body that evaluates the safety of food and food ingredients in the European Union. In 2012, EFSA concluded that bovine lactoferrin was safe as a novel food ingredient when added to infant formula at the level of 1000 mg/L. EFSA also estimated that the highest intake of bovine lactoferrin for infants would be 1.1 g/day [122]. The United States Food and Drug Administration (FDA) has approved that only bovine lactoferrin is considered generally recognized as safe for use in infant formula. This determination was made after reviewing scientific data and information on the safety of bovine lactoferrin in infant formula. The FDA has set a maximum amount of added bovine lactoferrin allowed in infant formula at 100 mg/100 g. This means that manufacturers of infant formula can add up to 100 mg of bovine lactoferrin per 100 g of formula without exceeding the FDA’s safety standards. Numerous randomized clinical trials have suggested that the FDA recommended dose of bovine lactoferrin in infant formula offers limited beneficial effect, while the added dosage reached at the level of 600 mg/kg/day or 850 mg/l induced desire effects [118, 123]. However, the specific dosage and its effects may vary depending on the study design and the population being studied. Based on the published literature, it was concluded that further in-depth research are required to determine the efficacy and safety of bovine lactoferrin supplementation in commercially available infant formula. The current low levels of lactoferrin supplement may not be sufficient to produce the desired effect, and it is important to evaluate the existing limits to ensure the recommended dose is accurate and effective.
Immunomodulatory Impacts of Lactoferrin
The immune system is a complex network of cells, tissues, and organs that work together to defend the body against harmful pathogens, such as bacteria, viruses, fungi, and parasites [124]. Inflammation is the tissue and body’s first biological response to harmful stimuli like tissue or cell injury, oxidative stress, pathogenic bacteria, and disease [10, 125]. Moreover, this defensive reaction of the body includes stimulating immune cells and activating chemical mediators such as cytokines and blood vessels that aid the removal of the inflammation. Some researchers and health organizations indicated that the regular consumption of phytochemicals and functional foods diminishes the occurrence of chronic diseases and inflammatory processes [11, 121, 126, 127]. Since the invention and molecular characterization of lactoferrin, several biological properties of this protein have been displayed in the literature. The immunomodulatory function of lactoferrin has been extensively explored and considered a key feature due to its nutritional benefits, gaining more attention in the pharmaceutical industry and research community. It is well known that lactoferrin is a powerful therapeutic agent to inhibit the growth and multiplication of pathogens, besides reducing inflammatory disorders in arthritis, allergy, and cancerous conditions [128, 129]. Siqueiros-Cendón et al. [130] suggested that lactoferrin significantly upregulated the adaptive and innate immune responses in neurodegenerative and inflammatory conditions by increasing lactoferrin secretion. Lactoferrin possesses anti-inflammatory and pro-inflammatory potential and can up- and downregulate endogenous inflammatory responses [131].
Earlier in vitro and in vivo investigations suggested that lactoferrin induces immunomodulatory responses via multiple mechanisms. It acts as an immunological mediator, naturally triggering both adaptive and innate immune responses [77], although the exact molecular mechanism of action behind the immunomodulatory lactoferrin influence has not been fully explored [128, 132]. According to Yen et al. [133], lactoferrin is a feedstuff additive that improves avian immunity. This finding highlights the effectiveness of the body’s natural immune defense mechanisms and suggests that secretory IgA and lysozyme may be able to prevent pathogen invasion from the gastrointestinal tract. Moreover, lactoferrin could work in conjunction with other bioactive compounds found in nature, including lysozyme, or it could prepare macrophages to eliminate E. coli in vivo [134]. Previously, it has been demonstrated that oral supplementation of bovine lactoferrin may have immune modulating properties and antioxidant activity in humans. The results showed significant increases in total T cell activation, helper T cell activation, cytotoxic T cell activation, and hydrophilic antioxidant capacity after supplementation with 200 mg of lactoferrin in health human [135]. Since 2020, with the outbreak of COVID-19 pandemic, it was reported that lactoferrin may have potential in preventing SARS-CoV-2 infection via preventing the entry of virus into host cell and improving the immunity of COVID-19 patients [136].
Badr et al. [137] measured the lysozyme and NO concentrations in the blood of broiler chicks experimentally challenged with E. coli and supplemented with lactoferrin in the ration. Lysozyme concentrations were increased numerically, but the NO had significantly larger levels compared to the non-supplemented lactoferrin group, indicating provoking of the non-specific immune response. Similarly, ovotransferrin (Otrf), an avian-like mammalian lactoferrin, significantly increased the generation and release of nitric oxide (NO) in MDCC-MSB1 cells, an avian lymphoblastoid cell line infected by Marek’s disease virus (MDV). A co-stimulatory impact of NO generation by IL-8 + lactoferrin and IFN-γ + Otrf was also noted [138]. NO has antiviral effects on many viruses, including MDV. This activity is owing to proteases with cysteine residues involved in viral replication and appear to be candidates for NO nitrosylation [138]. According to Hwang et al. [139], lactoferrin is a practical and efficient adjuvant that boosts the immune response to the Bacillus Calmette-Guerin vaccine and may even lessen tissue damage in the process.
In chickens immunized against the virus of infectious bursal disease (IBD), recombinant porcine lactoferrin dietary supplementation significantly elevated the expression of IL-12 and IFN-γ as well as augmented IBD-specific antibody titers and serum IgG values [140]. Additionally, recombinant porcine lactoferrin was supposed to work harmoniously with IFN-γ and IL-12 to enhance immune function. After an antigen is assaulted, recombinant porcine lactoferrin has been found to trigger producing adequate amounts of IL-12 and INF-γ, which may favor the formation of the Th1 lineage in lymphocyte subsets [140]. Lactoferrin thus suggests a potential function in a supplementary or alternative therapy against viral spreading, whether alone or in combination with cytokines.
Lactoferrin stimulated the antibody response via activating antigen-presenting cells (B-lymphocytes), which subsequently interacted with T-lymphocytes and promoted the T cell precursors’ maturation into competent helper cells [141]. Moreover, Dhennin-Duthille et al. [142] reported that the lactoferrin derived from bovine and human milk is capable of binding to surface receptors of T-lymphocytes (Jurkat cell line), and the molecular expression of lactoferrin receptors has also been found in all subsets of T-lymphocytes [130]. Additionally, lactoferrin can modulate the physiological functions, maturation, and migration of immune cells at the molecular level [143]. Furthermore, lactoferrin modulates the physiological functions of dendritic cells, initiating T cell activation [144]. Again, another clinical study showed that lactoferrin prevented sepsis via the neutralization of microbial antigens, thus effectively downregulating the pro-inflammatory effects [28].
Anti-inflammatory Impact of Lactoferrin
Recently, inflammation has been discussed as complex pathophysiological and physiological responses of the body to several non-infectious and infectious conditions. Although inflammation plays a decisive role in repairing tissues and eliminating pathogenic factors, when it is not controlled or restricted in time, it may be injurious and develop into severe systemic and chronic inflammatory cases. It is well known that inflammation produces important immune mediators such as chemokines and cytokines, which are responsible for stimulating innate immune cells, including macrophages, dendritic cells, and neutrophils, leading to the release and production of secondary immune mediators and consequently activating the adaptive immune response [145, 146]. Lactoferrin is categorized as a small family protein and acts as an “alarmin” released by immune cells during pathological conditions and plays a vital role in immunomodulation to control pathological and clinical situations [147, 148]. Lactoferrin can develop the conditional interaction between dendritic cells and neutrophils, activating innate and adaptive immune responses [149].
Furthermore, lactoferrin serves as a natural adjuvant, involving the modulation of immune responses against all pathophysiological conditions from infancy onwards. Mother milk contains abundant lactoferrin, which is responsible for providing protective immunity against infections during the initial stage of growth and development. There is a plethora of evidence in the literature that supports the protective effects of lactoferrin in various inflammatory cases. These findings are important for developing therapeutic tools and protocols to eliminate abnormal damage and treat specific diseases [150,151,152]. Okubo et al. [58] clarified that lactoferrin significantly inhibited releasing neutrophil extracellular traps (NETs) that are released during microbial infection and are involved in developing thrombosis and inflammatory diseases. Therefore, lactoferrin controls the release of NETs in autoimmune and other inflammatory conditions. Madkour et al. [153] assessed lactoferrin renoprotective effect against rats’ glycerol-induced rhabdomyolysis (RM). Administration of lactoferrin produced a robust and dose-dependent effect on Reno protection against RM. The primary mechanisms through which it exerts its renoprotective effects are its anti-inflammatory roles, as seen in the markedly decreased serum levels of IL-1β and kidney levels of CD68, NF-KB, NLRP3, and TXNIP; its antiapoptotic role, as seen in the significantly reduced kidney caspase-3 activity; and its concurrent antioxidant roles, as seen in its capacity to restore renal NRF2 expression. Consequently, lactoferrin can be recommended as an additional remedy and a strategy to lessen acute renal injury, especially in high-risk individuals. Numerous types of research examined the possibility that lactoferrin protects the intestine and may help avoid newborn intestinal inflammation. In the lipopolysaccharide (LPS)-induced acute abdominal inflammation model, lactoferrin utilizes its anti-inflammatory effects via reducing levels of the macrophage-related chemokines Ccl5 and Ccl2, as well as highly activated p65 signaling in lactoferrin supplemented mice, which is in charge of elevated levels of Ccl5 and Ccl2 expression by inhibiting NF-KB signaling. Thus, a lack of lactoferrin exacerbated the inflammatory response and encouraged macrophage chemotaxis to the site of inflammation [154].
Moreover, the impact of lactoferrin iron saturation (two lactoferrin forms: holo-lactoferrin and apo-lactoferrin) in preventing newborn LPS-induced intestinal inflammation as well as in vitro (primary intestinal epithelial cells) was examined [155]. The apo-lactoferrin outperformed the holo-lactoferrin in preventing colon mucosal inflammation and Gram-negative bacterial growth by decreasing the source of LPS and repairing mucosal injury by controlling the expression of the PFKFB3, PPAR-γ, and NF-KB genes. Fan and his co-authors investigated the protective effects of recombinant lactoferrin with different iron saturations (refers to the amount iron present in blood) on enteritis injury in young mice [155]. The results showed that rLF with higher iron saturation had a stronger protective effect on enteritis injury in young mice. Specifically, the mice treated with rLF with higher iron saturation had lower disease activity index scores, reduced intestinal inflammation, and lower levels of pro-inflammatory cytokines compared to the mice treated with rLF with lower iron saturation or no treatment Lactoferrin anti-inflammatory properties provide a new possible treatment for acute inflammatory illnesses [155].
Antioxidant Impact of Lactoferrin
Oxidative stress initiates the development of some chronic degenerative disorders like inflammation, cancer, neurodegenerative diseases, atherosclerosis, aging, and a defense against pathogens [156, 157]. Some pathophysiological and physiological conditions create abnormal levels of reactive oxygen species (ROS), which increases oxidative stress. Under natural physiological cases, the balance between ROS formation and their removal is controlled via various antioxidant enzymes, for instance, glutathione peroxidase, superoxide dismutase (SOD), and catalase. These enzymes convert the superoxide radicals into hydrogen peroxide radicals and transform reactive hydrogen peroxide into molecular oxygen and water [145].
Chelation of iron is the most important biological function of lactoferrin, which is essential for antimicrobial effects. It is also involved in antioxidant defense [158]. Inflamed tissues and cells release and produce an abundant amount of ROS, which has antimicrobial activities and causes tissue necrosis and apoptosis. Furthermore, injured tissue discharges ferrous and ferric iron that contributes to the Haber-Weiss reaction and releases unique free radicals. The iron-scavenging potential of lactoferrin strongly contributes to mitigating oxidative stress [159]. Antioxidant and cytoprotective influences of lactoferrin against oxidative stress induced by H2O2 were detected in human umbilical vein endothelial cells. Pretreatment of these cells with 25–100 μg/ml of lactoferrin declined the H2O2 cytotoxicity with increasing lactoferrin concentrations. Lactoferrin pretreatment at different levels also augmented the ferric-reducing antioxidant power value and decreased the H2O2 level in both extra- and intra-cellular assays [160]. Oral bovine lactoferrin (30, 100, 300 mg/kg) enormously improved antioxidant capacity and reduced the creation of ROS and blood pressure in dexamethasone-induced hypertension when used by 30 μg/kg/day subcutaneously [161]. On the other hand, lactoferrin is utilized to encapsulate polyphenolic flavonoids like quercetin as protein nanoparticles. For the creation of an easily scalable nanoparticles-in-protein gel, Raychaudhuri et al. [162] examined the interaction of quercetin with lactoferrin. This combination demonstrated a more substantial impact of the lactoferrin on the activity of antioxidant enzymes (SOD, catalase, and malondialdehyde).
Anticancer Effects of Lactoferrin
Cancer is a multi-mechanism chronic procedure that leads to the uncontrolled and abnormal proliferation of cells owing to many unknown and etiological elements. The cancerous cells rapidly invade other tissues and develop a generalized case. It has been displayed that lactoferrin-derived peptides, including LfcinB-CLICK and LFcinB, cure cancerous conditions [163]. Li et al. [164] investigated the anticancer influence of lactoferrin on tumors HT29 and HCT8 and colon cancer cells. The results indicated that lactoferrin (5–100 mg/ml) inhibited cell survival and migration by downregulating VEGFA, VEGFR2, pPI3K, pErK1/2, and pAKt protein expression. Li et al. [165] tested bovine lactoferrin in a cell model of esophageal cancer. Lactoferrin decreased the viability, motility, and invasion of KYSE450 cells and triggered apoptosis, whether used alone or in conjunction with linolenic acid. Through lowering lithocholyltaurine levels and consequently preventing the phosphorylation of JAK2/STAT3/Erk/AKT proteins, the combination of linolenic acid and lactoferrin demonstrated an additive impact in decreasing KYSE450 tumor growth.
Earlier studies suggested that bovine lactoferrin inhibited the plasma membrane V-ATPase activity, leading to a decrease in the tumor microenvironment acidity and, ultimately, suppression of cancer metastasis and growth [166, 167]. Furthermore, lactoferrin-mediated apoptosis in cancer cells has also been revealed to be indirectly associated with the inhibition of apoptosis inhibitors. Numerous cancer cells contain survivin, which is responsible for inhibiting the caspase effect, causing cancer to evade apoptosis [168]. Lactoferrin significantly decreases the expression of the Survivin gene. Nano-formulated bovine lactoferrin also inhibited the anti-Survivin activity in colon CSCs and tumors [168]. Lactoferrin enhances the caspase cleavage, especially caspases 3, 8, and 9, by increasing the concentration of mitochondrial protease inhibitors IAP, HTRA2, and SMAC. However, the apoptosis induction in cancer cells by lactoferrin was mainly attributed to the IAP protease inhibition [169]. Lactoferrin increases the sensitivity of chemo-resistant tumor cells by swift internalization and increases the sensitivity to antitumor drugs (doxorubicin), thus overcoming chemoresistance [170]. Recently, a lactoferrin nanocarrier significantly increased the expression level of the cytokines IFN- and TNF-, improving anticancer activity [171]. Furthermore, iron-saturated lactoferrin demonstrated more substantial anticancer effects than regular lactoferrin [171].
Antibacterial Effects of Lactoferrin
The antimicrobial potential of lactoferrin is mainly attributed to its ability to bind to iron and make it unavailable to bacteria. Lactoferrin is a critical constituent of the immunomodulatory response; the antibacterial role of lactoferrin is a key element of the innate immune response of animals and humans [172]. Lactoferrin shows antimicrobial effects against many pathogens, including fungi, parasites, viruses, and bacteria, via two different mechanisms [173, 174]: (i) the antibacterial mechanism of action of lactoferrin varies depending on its iron saturation state. In holo-lactoferrin, the iron is sequestered in the protein’s binding sites, preventing bacteria from accessing it. This deprives the bacteria of a vital nutrient, which can limit their growth and survival. Additionally, the iron-binding sites in lactoferrin have been shown to bind to bacterial cell walls, disrupting their integrity and leading to bacterial death. (ii) Bactericidal lactoferrin activity is primarily attributed to bacterial cell membrane damage caused by direct interaction with the highly cationic N-terminus with negatively charged LPS of Gram-negative bacteria [175].
It is well documented that lactoferrin shows antibacterial potential against numerous bacteria such as Y. pestis, S. canis, S. pyogenes, Proteus sp., S. agalactiae, S. zooepidemicus, K. pneumoniae, and C. albicans [18, 176]. Dietary lactoferrin supplementation effectively reduces the danger of necrotizing enterocolitis by manipulating the gastric microbiota in immature infants [177]. Moreover, synthetic lactoferrin peptides induce bactericidal action by damaging the bacterial membrane, followed by intracellular internalization [178]. Furthermore, the therapeutic application of lactoferrin downregulated the expression of the luxS gene, inhibiting the biofilm formation [179]. Bellés et al. [180] found that native and iron-saturated lactoferrin restored the average numbers of these bacteria and TLR expression in a mouse model of intestinal dysbiosis caused by clindamycin, where Bacteroidaceae, Prevotellaceae, and Rikenellacea were diminished. Therefore, to make functional food, lactoferrin might be an excellent element to use. Al-Nabulsi and Holley [181] reported the synergistic bactericidal effects of bovine lactoferrin, sodium lactate, and NaCl as a chelating agent against E. coli 0157:H7 stain. Similarly, another study demonstrated the synergistic antibacterial effects of human apo-lactoferrin and lysozyme enzymes against S. pneumoniae. Results indicated that apo-lactoferin and lysozyme had a robust bactericidal effect compared to the lysozyme alone, reducing bacterial multiplication [182]. Furthermore, a novel recombinant mouse lactoferrin was tested against methicillin-resistant Staphylococcus aureus (MRSA) in the mouse peritonitis model. The results showed that recombinant mouse lactoferrin improved immune response by lowering the bacterial load and decreasing cytokine concentrations such as IL-17 and IL-6 in post-challenge mice [183]. Similarly, Redwan et al. [184] investigated the synergistic bactericidal influences of camel lactoferrin along with antibiotics against MRSA and observed that camel lactoferrin in combination with oxacillin or vancomycin significantly increased the antibacterial response against MRSA in comparison with each agent alone. Finally, lactoferrin antimicrobial efficacy is tested against human pathogens and applied in agriculture and crop sciences [185]. The plants were challenged with Ps. Syringae pv. tabaci and Botrytis cinereal revealed that lactoferrin increased transgenic plants’ survival rate up to 30 days after infection, while the wild-type plants died due to pathogen injury.
Additionally, the minimum inhibitory level of lactoferrin and gellan gum complex nanoparticles (9:1 particles) against E. coli and S. aureus was six times lower (0.3 mg/ml) than the concentration of pure lactoferrin (2 mg/ml). In the presence of carboxymethyl cellulose, lactoferrin nanoparticles on strawberries increased fruit shelf-life by up to 6 days. Therefore, it was determined that lactoferrin gellan gum complexation was a potential method for enhancing lactoferrin’s antibacterial effect and expanding its use as a food preservative [186]. Lactoferrin was identified as an effective prebiotic feed additive by 0.1 g/kg that can be used for prophylaxis or treatment of chicks experimentally challenged with multidrug-resistant E. coli [137]. In this study, lactoferrin improved chick performance, prevented death and clinical signs, and eliminated the bacteria from the liver. Bovine lactoferrin has also been reported to eliminate the bacterial infection caused by E. coli in cattle [187]. Yen et al. [188] experimented on mice by infecting them with pathogenic strains of S. aureus, E. coli, and Candida albicans and giving them milk fortified with porcine lactoferrin. This porcine lactoferrin demonstrated broad-spectrum antibacterial activity in the intestine; markedly enhanced weight growth; lowered bacterial counts in the liver, blood, and intestinal fluid; and improved microvilli health in the alveoli in the lungs and small intestine. Using the expression system based on polyethylene terephthalate, a chimeric peptide from camel lactoferrin was created in the periplasmic space of Escherichia coli and demonstrated antibacterial influences on both Gram-negative and Gram-positive avian pathogen bacteria (S. aureus, P. aeruginosa, S. epidermidis), and avian pathogenic E. coli and S. enteritidis, while having little hemolysis activity in chicken blood and high serum stability [189].
Thus, it is recommended that using lactoferrin either by itself or in conjunction with antibiotics is a crucial tactic for treating bacterial infections, particularly those brought on by resistant strains. Additionally, purified lactoferrin was digested with pepsin to produce peptide extracts (cationic peptide extract (CPE) and total peptide extract (TPE)) and showed antibacterial activity against avian pathogens (S. typhimurium, S. enteritidis, two E. coli strains, TK3 O1:K1 and O78:H80, and S. aureus). The most effective extract was lactoferrin CPE, which killed four strains at levels of 2.5 and 5 mg/ml (viability equal 0), and S. Typhimurium viability only reached 288 log CFU/ml with 5 mg CPE/ml. In addition, for TPE to have equivalent bactericidal effects as CPE, its concentration needed to be two to four times higher [190]. Lactoferrin also contains several natural peptides that have been shown to have potent antibacterial activity. These peptides include lactoferricin-, lactoferrampin-, and lactoferrin-derived peptides. These peptides showed strong antibacterial effects against a wide range of bacteria, including Gram-positive and Gram-negative bacteria, as well as antibiotic-resistant strains. It has been reported that lactoferricin and lactoferrampin have powerful antibacterial effect against various foodborne pathogens such as Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus [191]. These peptides have also been shown to have antiviral activity against several viruses, including herpes simplex virus type 1 and type 2, and human cytomegalovirus [192].
Bovine lactoferrin, human lactoferrin, and its avian homolog ovotransferrin were found to have an anti-chlamydial action [193]. According to their findings, ovotransferrin and lactoferrin have bactericidal effects on extracellular Chlamydophila Psittaci (Cp.), an obligate intracellular pathogen, which reduced the infection rate in HD11 chicken macrophages and Buffalo Green Monkey kidney cells, which were used as artificial hosts. Ovotransferrin, however, has had a more significant impact on Cp. Psittaci’s demise. Furthermore, since cell infection was stopped at a concentration ten times lower than that of human and bovine lactoferrin, voTF’s non-bactericidal dose was more efficient in blocking irreversible bacterial attachment and cell entrance to HD11 chicken macrophages. Nevertheless, transferrins did not appear to have any impact on intracellular replication. The investigators speculated that ovoTF, which is only found in birds, may be better suited to annihilating avian infections because they could not explain the differences in the anti-chlamydial action of transferrins. According to this study, transferrins may help prevent Cp. psittaci infection in birds with high zoonotic potential.
Antiviral Effects of Lactoferrin
Lactoferrin exhibits a sturdy antiviral response against a wide array of enveloped and naked RNA and DNA viruses, including HCV, HIV, HBV, HPV, influenza virus, and poliovirus, through direct binding with virus surfaces and iron competition or scavenging for binding with host cells [194,195,196]. Pups consuming recombinant porcine lactoferrin–enriched milk had a considerably more significant survival percentage and reduced viral genomic RNA content after being inoculated with enterovirus type 71 (EV71) 4 days after birth, demonstrating that porcine lactoferrin had a blocking influence on viral infection EV71 [197]. It has been proposed that lactoferrin prevents the cellular internalization of virus particles in host cells either by direct attachment to the virus particles or by blocking the cellular receptors [198]. Lactoferrin directly inhibits the entry of some viruses into host cells, including HIV, herpes simplex virus, human papillomavirus, and rotavirus [30, 199, 200]. Additionally, it was mentioned that lactoferrin could inhibit the caspase-3 response and mitigate the host apoptosis induced by the influenza virus [201].
Moreover, lactoferrin neutralizes the Adenovirus infection via binding to viral IIIa and III polypeptides. However, the anti-HCV effect of lactoferrin is attributed to decreasing the RNA titer, indicating the active use of lactoferrin as an adjuvant remedy for interferon and ribavirin in treating HCV [202, 203]. In an in vitro study using hepatocytes (HepG2 cell line) infected with HBV and observing that the treatment with lactoferrin significantly reduced the multiplication of the virus, it was suggested that lactoferrin might apply for anti-hepatitis B virus therapy [204]. On the other hand, lactoferrin inhibits interleukin-6 synthesis, which is involved in disorders of iron homeostasis and leads to an excess of intracellular iron, which is favorable to viral infection and replication. The known antiviral action of lactoferrin has been established against DNA, RNA, and naked and enveloped viruses, and lactoferrin may be effective in combating SARS-CoV-2 infection besides other viral infections [205]. Lactoferrin plays a significant role in improving immunity and has moderate influences on the infection of SARS-CoV-2 [206]. Lactoferrin have been demonstrated to inhibit the infection of SARS-CoV-2 in all cell models in the nanomolar range. These mechanisms include blocking virus binding to cellular heparan sulfate and enhancing interferon response [207].
Conclusion
Due to the emergence of antibiotic resistance, the utilization of nutraceuticals is gaining more attention as an alternative strategy for diverse biomedical applications. Lactoferrin is a natural iron-binding glycoprotein and cell secretory mediator extensively present in bovine milk and other exocrine secretions of humans and animals. Lactoferrin is also known for its potent antibacterial, antiviral, antioxidant, anti-inflammatory, immune-modulatory, and anticancer alternatives with integrated inhibitory paths similar to the effects of synthetic chemotherapeutic medicines and minimum adverse effects. Lactoferrin has the potential to boost the immune response against numerous pathological conditions caused by infectious and non-infectious agents. Interest in exploring the therapeutic potential of lactoferrin among researchers has increased due to its multiple medical applications.
Data Availability
The authors acknowledge that the data presented in this study must be deposited and made publicly available in an acceptable repository before publication.
References
Chamorro MF, Cernicchiaro N, Haines DM (2017) Evaluation of the effects of colostrum replacer supplementation of the milk replacer ration on the occurrence of disease, antibiotic therapy, and performance of pre-weaned dairy calves. J Dairy Sci 100(2):1378–1387
Saeed M, Babazadeh D, Arif M, Arain M, Bhutto Z, Shar A, Kakar M, Manzoor R, Chao S (2017) Silymarin: a potent hepatoprotective agent in poultry industry. Worlds Poult Sci J 73(3):483–492
Sun Y, Rajput IR, Arain MA, Li Y, Baloch DM (2017) Oral administration of Saccharomyces boulardii alters duodenal morphology, enzymatic activity and cytokine production response in broiler chickens. Anim Sci J 88(8):1204–1211
Alagawany M, Abd El-Hack ME, Saeed M, Naveed M, Arain MA, Arif M, Tiwari R, Khandia R, Khurana SK, Karthik K (2020) Nutritional applications and beneficial health applications of green tea and l-theanine in some animal species: a review. J Anim Physiol Anim Nutr 104(1):245–256
Arain MA, Mei Z, Hassan F, Saeed M, Alagawany M, Shar A, Rajput I (2018) Lycopene: a natural antioxidant for prevention of heat-induced oxidative stress in poultry. Worlds Poult Sci J 74(1):89–100
Nabi F, Arain M, Hassan F, Umar M, Rajput N, Alagawany M, Syed S, Soomro J, Somroo F, Liu J (2020) Nutraceutical role of selenium nanoparticles in poultry nutrition: a review. Worlds Poult Sci J 76(3):459–471
Nabi F, Arain MA, Rajput N, Alagawany M, Soomro J, Umer M, Soomro F, Wang Z, Ye R, Liu J (2020) Health benefits of carotenoids and potential application in poultry industry: a review. J Anim Physiol Anim Nutr 104(6):1809–1818
Pirzado SA, Arain MA, Huiyi C, Fazlani SA, Alagawany M, Gouhua L (2021) Effect of Azomite on growth performance, immune function and tibia breaking strength of broiler chickens during starter period. Anim Biotechnol 33(7):1539–1544
Rajput IR, Ying H, Yajing S, Arain MA, Weifen L, Ping L, Bloch DM, Wenhua L (2017) Saccharomyces boulardii and Bacillus subtilis B10 modulate TLRs and cytokines expression patterns in jejunum and ileum of broilers. PLoS One 12(3):e0173917
Saeed M, Babazadeh D, Naveed M, Arain MA, Hassan FU, Chao S (2017) Reconsidering betaine as a natural anti-heat stress agent in poultry industry: a review. Trop Anim Health Prod 49:1329–1338
Saeed M, Naveed M, Arain M, Arif M, Abd El-Hack M, Alagawany M, Siyal F, Soomro R, Sun C (2017) Quercetin: nutritional and beneficial effects in poultry. Worlds Poult Sci J 73(2):355–364
Saeed M, Naveed M, BiBi J, Kamboh AA, Arain MA, Shah QA, Alagawany M, El-Hack ME, Abdel-Latif MA, Yatoo M (2018) The promising pharmacological effects and therapeutic/medicinal applications of punica granatum L.(Pomegranate) as a functional food in humans and animals. Recent Patents Inflamm Allergy Drug Discov 12(1):24–38
Arain MA, Nabi F, Marghazani IB, Hassan FU, Soomro H, Kalhoro H, Soomro F, Buzdar JA (2022) In ovo delivery of nutraceuticals improves health status and production performance of poultry birds: a review. World’s Poult Sci J 78(3):765–788
Changxing L, Chenling M, Alagawany M, Jianhua L, Dongfang D, Gaichao W, Wenyin Z, Syed S, Arain M, Saeed M (2018) Health benefits and potential applications of anthocyanins in poultry feed industry. Worlds Poult Sci J 74(2):251–264
Arif M, Alagawany M, Abd El-Hack M, Saeed M, Arain M, Elnesr S (2019) Humic acid as a feed additive in poultry diets: a review. Iranian J Vet Res 20(3):167
Arain MA, Khaskheli GB, Shah AH, Marghazani IB, Barham GS, Shah QA, Khand FM, Buzdar JA, Soomro F, Fazlani SA (2022) Nutritional significance and promising therapeutic/medicinal application of camel milk as a functional food in human and animals: a comprehensive review. Anim Biotechnol 1–18. https://doi.org/10.1080/10495398.2022.2059490
Nabi F, Arain MA, Fazlani SA, Khalid M, Bugti F, Ali S, Fareed SK, Liu J (2022) Effect of in ovo trace element supplementation on immune-related cells of the small intestine of post-hatched broiler chicken. Biol Trace Elem Res 1–10. https://doi.org/10.1007/s12011-022-03492-0
González-Chávez SA, Arévalo-Gallegos S, Rascón-Cruz Q (2009) Lactoferrin: structure, function and applications. Int J AntimicrobAgents 33(4):301. e1-301. e8
Superti F (2020) Lactoferrin from bovine milk: a protective companion for life. Nutrients 12(9):2562
Steijns JM, Van Hooijdonk A (2000) Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br J Nutr 84(S1):11–17
Inoue M, Yamada J, Kitamura N, Shimazaki K-I, Andrén A, Yamashita T (1993) Immunohistochemical localization of lactoferrin in bovine exocrine glands. Tissue Cell 25(5):791–797
Lönnerdal B (2003) Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr 77(6):1537S-1543S
Pan Y, Rowney M, Guo P, Hobman P (2007) Biological properties of lactoferrin: an overview. Australian J Dairy Technol 62(1):31
Vogel HJ (2012) Lactoferrin, a bird’s eye view. Biochem Cell Biol 90(3):233–244
Jia Y, Lu Y, Wang X, Yang Y, Zou M, Liu J, Jin W, Wang X, Pang G, Huang L (2021) Mass spectrometry based quantitative and qualitative analyses reveal N-glycan changes of bovine lactoferrin at different stages of lactation. LWT 147:111626
Luna-Castro S, Samaniego-Barrón L, Serrano-Rubio L, Ceballos-Olivera I, Avalos-Gómez C, de la Garza M (2017) Lactoferrin: a powerful antimicrobial protein present in milk. J Adv Dairy Res 5(4):1000195. https://doi.org/10.4172/2329-888X.1000195
Kell DB, Heyden EL, Pretorius E (2020) The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front Immunol 11:1221
Majka G, Więcek G, Śróttek M, Śpiewak K, Brindell M, Koziel J, Marcinkiewicz J, Strus M (2016) The impact of lactoferrin with different levels of metal saturation on the intestinal epithelial barrier function and mucosal inflammation. Biometals 29:1019–1033
Moreno-Expósito L, Illescas-Montes R, Melguizo-Rodríguez L, Ruiz C, Ramos-Torrecillas J, de Luna-Bertos E (2018) Multifunctional capacity and therapeutic potential of lactoferrin. Life Sci 195:61–64
Drobni P, Näslund J, Evander M (2004) Lactoferrin inhibits human papillomavirus binding and uptake in vitro. Antiviral Res 64(1):63–68
Chen J-M, Fan Y-C, Lin J-W, Chen Y-Y, Hsu W-L, Chiou S-S (2017) Bovine lactoferrin inhibits dengue virus infectivity by interacting with heparan sulfate, low-density lipoprotein receptor, and DC-SIGN. Int J Mol Sci 18(9):1957
Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, Mazurier J (2008) Lactoferrin structure and functions. Bioact Components Milk 606:163–194
Abd El Hafez SM, Ismael AB, Mahmoud MB, Elaraby A-KA (2013) Development of new strategy for non-antibiotic therapy: bovine lactoferrin has a potent antimicrobial and immunomodulator effects. Adv Infect Dis 3(3):185–192
Zarzosa-Moreno D, Avalos-Gómez C, Ramírez-Texcalco LS, Torres-López E, Ramírez-Mondragón R, Hernández-Ramírez JO, Serrano-Luna J, de la Garza M (2020) Lactoferrin and its derived peptides: an alternative for combating virulence mechanisms developed by pathogens. Molecules 25(24):5763
Sorensen M, Sorensen S (1940) The proteins in whey. Compte rendu des Travaux du Laboratoire de Carlsberg Ser Chim 23(7):55–99
Anderson BF, Baker HM, Norris GE, Rice DW, Baker EN (1989) Structure of human lactoferrin: crystallographic structure analysis and refinement at 2· 8 Å resolution. J Mol Biol 209(4):711–734
Karav S, German JB, Rouquié C, Le Parc A, Barile D (2017) Studying lactoferrin N-glycosylation. Int J Mol Sci 18(4):870
Karav S (2018) Selective deglycosylation of lactoferrin to understand glycans’ contribution to antimicrobial activity of lactoferrin. Cell Mol Biol (Noisy-le-grand) 64(9):52–57
Montagne P, Cuilliere M, Mole C, Bene M, Faure G (2001) Changes in lactoferrin and lysozyme levels in human milk during the first twelve weeks of lactation. Bioact Components Hum Milk. 501:241–247
Yang Z, Jiang R, Chen Q, Wang J, Duan Y, Pang X, Jiang S, Bi Y, Zhang H, Lönnerdal B (2018) Concentration of lactoferrin in human milk and its variation during lactation in different Chinese populations. Nutrients 10(9):1235
Kehoe S, Jayarao B, Heinrichs A (2007) A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. J Dairy Sci 90(9):4108–4116
Konuspayeva G, Faye B, Loiseau G, Levieux D (2007) Lactoferrin and immunoglobulin contents in camel’s milk (Camelus bactrianus, Camelus dromedarius, and Hybrids) from Kazakhstan. J Dairy Sci 90(1):38–46
Hiss S, Meyer T, Sauerwein H (2008) Lactoferrin concentrations in goat milk throughout lactation. Small Rumin Res 80(1–3):87–90
Czosnykowska-Łukacka M, Orczyk-Pawiłowicz M, Broers B, Królak-Olejnik B (2019) Lactoferrin in human milk of prolonged lactation. Nutrients 11(10):2350
Sanchez L, Aranda P, Pérez M, Calvo M (1988) Concentration of lactoferrin and transferrin throughout lactation in cow’s colostrum and milk. Biol Chemist 369(2):1005–1008
Conesa C, Sánchez L, Rota C, Pérez M-D, Calvo M, Farnaud S, Evans RW (2008) Isolation of lactoferrin from milk of different species: calorimetric and antimicrobial studies. Comp Biochem Physiol B: Biochem Mol Biol 150(1):131–139
Giacinti G, Basiricò L, Ronchi B, Bernabucci U (2013) Lactoferrin concentration in buffalo milk. Italian J Anim Sci 12(1):e23
Konuspayeva G, Serikbayeva A, Loiseau G, Narmuratova M, Faye B (2005) Lactoferrin of camel milk of Kazakhstan. Desertification Combat Food Safety: Added Value Camel Prod 362:158–167
Balasubramanian SA, Pye DC, Willcox MDP (2012) Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp Eye Res 96(1):132–137
Hashem NM, El-Son MA, Ateya AI, Saleh RM (2022) Impact of lactoferrin supplementation on oxidative stress, gene expression and immunity dysfunction induced by Aeromonas veronii in Nile tilapia (Oreochromis niloticus). Aquac Res 53(6):2392–2407
Khan FB, Anwar I, Redwan EM, Palakkott A, Ashraf A, Kizhakkayil J, Iratni R, Maqsood S, Ayoub MA (2022) Camel and bovine milk lactoferrins activate insulin receptor and its related AKT and ERK1/2 pathways. J Dairy Sci 105(3):1848–1861
Yoshida S, Wei Z, Shinmura Y, Fukunaga N (2000) Separation of lactoferrin-a and-b from bovine colostrum. J Dairy Sci 83(10):2211–2215
Hirai Y, Kawakata N, Satoh K, Ikeda Y, Hisayasu S, Orimo H, Yoshino Y (1990) Concentrations of lactoferrin and iron in human milk at different stages of lactation. J Nutr Sci Vitaminol 36(6):531–544
Cornish J, Callon KE, Naot D, Palmano KP, Banovic T, Bava U, Watson M, Lin J-M, Tong P, Chen Q (2004) Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 145(9):4366–4374
Dierick M, Vanrompay D, Devriendt B, Cox E (2021) Lactoferrin, a versatile natural antimicrobial glycoprotein that modulates the host’s innate immunity. Biochem Cell Biol 99(1):61–65
Arnold R, Brewer M, Gauthier J (1980) Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect Immun 28(3):893–898
Wisgrill L, Wessely I, Spittler A, Förster-Waldl E, Berger A, Sadeghi K (2018) Human lactoferrin attenuates the proinflammatory response of neonatal monocyte-derived macrophages. Clin Exp Immunol 192(3):315–324
Okubo K, Kamiya M, Urano Y, Nishi H, Herter JM, Mayadas T, Hirohama D, Suzuki K, Kawakami H, Tanaka M (2016) Lactoferrin suppresses neutrophil extracellular traps release in inflammation. EBioMedicine 10:204–215
Hara K, Ikeda M, Saito S, Matsumoto S, Numata K, Kato N, Tanaka K, Sekihara H (2002) Lactoferrin inhibits hepatitis B virus infection in cultured human hepatocytes. Hepatol Res 24(3):228–235
Redwan ERM, Tabll A (2007) Camel lactoferrin markedly inhibits hepatitis C virus genotype 4 infection of human peripheral blood leukocytes. J Immunoassay Immunochem 28(3):267–277
Agrawal RP, Dogra R, Mohta N, Tiwari R, Singhal S, Sultania S (2009) Beneficial effect of camel milk in diabetic nephropathy. Acta Biomed 80(2):131–134
Kanwar JR, Palmano KP, Sun X, Kanwar RK, Gupta R, Haggarty N, Rowan A, Ram S, Krissansen GW (2008) ‘Iron-saturated’lactoferrin is a potent natural adjuvant for augmenting cancer chemotherapy. Immunol Cell Biol 86(3):277–288
Kanwar JR, Mahidhara G, Roy K, Sasidharan S, Krishnakumar S, Prasad N, Sehgal R, Kanwar RK (2015) Fe-bLf nanoformulation targets survivin to kill colon cancer stem cells and maintains absorption of iron, calcium and zinc. Nanomedicine 10(1):35–55
Iigo M, Alexander DB, Xu J, Futakuchi M, Suzui M, Kozu T, Akasu T, Saito D, Kakizoe T, Yamauchi K (2014) Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine. Biometals 27:1017–1029
Gupta I, Sehgal R, Kanwar RK, Punj V, Kanwar JR (2015) Nanocapsules loaded with iron-saturated bovine lactoferrin have antimicrobial therapeutic potential and maintain calcium, zinc and iron metabolism. Nanomedicine 10(8):1289–1314
Latorre D, Pulvirenti N, Covino DA, Varano B, Purificato C, Rainaldi G, Gauzzi MC, Fantuzzi L, Conti L, Donninelli G (2015) Bovine lactoferrin-induced CCL1 expression involves distinct receptors in monocyte-derived dendritic cells and their monocyte precursors. Toxins 7(12):5472–5483
Bertuccini L, Costanzo M, Iosi F, Tinari A, Terruzzi F, Stronati L, Aloi M, Cucchiara S, Superti F (2014) Lactoferrin prevents invasion and inflammatory response following E. coli strain LF82 infection in experimental model of Crohn’s disease. Dig Liver Dis 46(6):496–504
Abbas Z, Doosh K, Yaseen N (2019) Effect of purified goat milk lactoferrin on cancer cell growth (AMN-3) in vitro. Biochem Cell Arch 19(Suppl. 1):2661–2667
Arain MA, Nabi F, Shah QA, Alagawany M, Fazlani SA, Khalid M, Soomro F, Khand FM, Farag MR (2022) The role of early feeding in improving performance and health of poultry: herbs and their derivatives. Worlds Poult Sci J 78(2):499–513
Nabi F, Arain MA, Bhutto ZA, Shah QA, Bangulzai N, Ujjan NA, Fazlani SA (2022) Effect of early feeding of L-arginine and L-threonine on hatchability and post-hatch performance of broiler chicken. Trop Anim Health Prod 54(6):380
Pirzado SA, Hassan FU, Arain MA, Zhengke W, Huiyi C, Haile TH, Guohua L (2021) Effect of azomite on growth performance, nutrient utilization, serum biochemical index and bone mineralization of broilers fed low protein diet. Italian J Anim Sci 20(1):1282–1291
Saeed M, Arain MA, Ali Fazlani S, Marghazani IB, Umar M, Soomro J, Bhutto ZA, Soomro F, Noreldin AE, Abd El-Hack ME (2021) A comprehensive review on the health benefits and nutritional significance of fucoidan polysaccharide derived from brown seaweeds in human, animals and aquatic organisms. Aquac Nutr 27(3):633–654
Saeed M, Babazadeh D, Naveed M, Alagawany M, Abd El-Hack ME, Arain MA, Tiwari R, Sachan S, Karthik K, Dhama K (2019) In ovo delivery of various biological supplements, vaccines and drugs in poultry: current knowledge. J Sci Food Agric 99(8):3727–3739
Iglesias-Figueroa BF, Espinoza-Sánchez EA, Siqueiros-Cendón TS, Rascón-Cruz Q (2019) Lactoferrin as a nutraceutical protein from milk, an overview. Int Dairy J 89:37–41
Borodina I, Kenny LC, McCarthy CM, Paramasivan K, Pretorius E, Roberts TJ, van der Hoek SA, Kell DB (2020) The biology of ergothioneine, an antioxidant nutraceutical. Nutr Res Rev 33(2):190–217
Konstanti P, van Splunter M, van den Brink E, Belzer C, Nauta A, van Neerven RJ, Smidt H (2022) The effect of nutritional intervention with lactoferrin, galactooligosacharides and vitamin D on the gut microbiota composition of healthy elderly women. Nutrients 14(12):2468
Actor JK, Hwang S-A, Kruzel ML (2009) Lactoferrin as a natural immune modulator. Curr Pharm Des 15(17):1956–1973
Saki A, Mahmoudi H (2015) Effects of in ovo injection of bovine lactoferrin before incubation in layer breeder eggs on tibia measurements and performance of laying hens. Animal 9(11):1813–1819
Humphrey BD, Huang N, Klasing KC (2002) Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. J Nutr 132(6):1214–1218
Kawakami H, Park H, Park S, Kuwata H, Shephard R, Aoyagi Y (2015) Effects of enteric-coated lactoferrin supplementation on the immune function of elderly individuals: a randomised, double-blind, placebo-controlled trial. Int Dairy J 47:79–85
Takeuchi T, Jyonotsuka T, Kamemori N, Kawano G, Shimizu H, Ando K, Harada E (2006) Enteric-formulated lactoferrin was more effectively transported into blood circulation from gastrointestinal tract in adult rats. Exp Physiol 91(6):1033–1040
Conesa C, Calvo M, Sánchez L (2010) Recombinant human lactoferrin: a valuable protein for pharmaceutical products and functional foods. Biotechnol Adv 28(6):831–838
Krolitzki E, Schwaminger SP, Pagel M, Ostertag F, Hinrichs J, Berensmeier S (2022) Current practices with commercial scale bovine lactoferrin production and alternative approaches. Int Dairy J 126:105263
Terpe K (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 72:211–222
Liu T, Zhang YZ, Wu XF (2006) Recombinant functional human lactoferrin expressed in baculovirus system. Acta biochimica et biophysica Sinica 38(3):201–206
Tutykhina I, Bezborodova O, Verkhovskaya L, Shmarov M, Logunov DY, Nemtsova E, Naroditskii B, Yakubovskaya R, Ginzburg A (2009) Recombinant pseudoadenovirus nanostructure with the human lactoferrin gene: production and study of lactoferrin expression and properties in vivo. Mol Genet Microbiol Virol 24:32–36
Liang Q, Richardson T (1993) Expression and characterization of human lactoferrin in yeast Saccharomyces cerevisiae. J Agric Food Chem 41(10):1800–1807
Choi B-K, Actor JK, Rios S, d’Anjou M, Stadheim TA, Warburton S, Giaccone E, Cukan M, Li H, Kull A (2008) Recombinant human lactoferrin expressed in glycoengineered Pichia pastoris: effect of terminal N-acetylneuraminic acid on in vitro secondary humoral immune response. Glycoconj J 25:581–593
Nakamura I, Watanabe A, Tsunemitsu H, Lee N-Y, Kumura H, Shimazaki K-I, Yagi Y (2001) Production of recombinant bovine lactoferrin N-lobe in insect cells and its antimicrobial activity. Protein Expr Purif 21(3):424–431
Hyvönen P, Suojala L, Haaranen J, Von Wright A, Pyörälä S (2006) Human and bovine lactoferrins in the milk of recombinant human lactoferrin-transgenic dairy cows during lactation. Biotechnol J: Healthc Nutr Technol 1(4):410–412
Yang P, Wang J, Gong G, Sun X, Zhang R, Du Z, Liu Y, Li R, Ding F, Tang B (2008) Cattle mammary bioreactor generated by a novel procedure of transgenic cloning for large-scale production of functional human lactoferrin. PloS one 3(10):e3453
Han Z-S, Li Q-W, Zhang Z-Y, Xiao B, Gao D-W, Wu S-Y, Li J, Zhao H-W, Jiang Z-L, Hu J-H (2007) High-level expression of human lactoferrin in the milk of goats by using replication-defective adenoviral vectors. Protein Expr Purif 53(1):225–231
Zhang J, Li L, Cai Y, Xu X, Chen J, Wu Y, Yu H, Yu G, Liu S, Zhang A (2008) Expression of active recombinant human lactoferrin in the milk of transgenic goats. Protein Expr Purif 57(2):127–135
Li L, Shen W, Min L, Dong H, Sun Y, Pan Q (2006) Human lactoferrin transgenic rabbits produced efficiently using dimethylsulfoxide–sperm-mediated gene transfer. Reprod Fertil Dev 18(6):689–695
Zhao C, Liu Z, Fan B, Dai Y, Wang L, Zheng M, Wang M, Niu H, Xi F, Li N (2006) Differential glycosylation of rhLf expressed in the mammary gland of transgenic mice. Anim Biotechnol 17(1):13–20
Liu T, Zhang Y-Z, Wu X-F (2005) High level expression of functionally active human lactoferrin in silkworm larvae. J Biotechnol 118(3):246–256
Nandi S, Suzuki YA, Huang J, Yalda D, Pham P, Wu L, Bartley G, Huang N, Lönnerdal B (2002) Expression of human lactoferrin in transgenic rice grains for the application in infant formula. Plant Sci 163(4):713–722
Rachmawati D, Mori T, Hosaka T, Takaiwa F, Inoue E, Anzai H (2005) Production and characterization of recombinant human lactoferrin in transgenic Javanica rice. Breed Sci 55(2):213–222
Salmon V, Legrand D, Slomianny M-C, El Yazidi I, Spik G, Gruber V, Bournat P, Olagnier B, Mison D, Theisen M (1998) Production of human lactoferrin in transgenic tobacco plants. Protein Expr Purif 13(1):127–135
Chong DK, Langridge WH (2000) Expression of full-length bioactive antimicrobial human lactoferrin in potato plants. Transgenic Res 9(1):71
Lee T-J, Coyne DP, Clemente TE, Mitra A (2002) Partial resistance to bacterial wilt in transgenic tomato plants expressing antibacterial lactoferrin gene. J Am Soc Hortic Sci 127(2):158–164
Wakabayashi H, Yamauchi K, Takase M (2006) Lactoferrin research, technology and applications. Int Dairy J 16(11):1241–1251
Stowell K, Rado T, Funk W, Tweedie J (1991) Expression of cloned human lactoferrin in baby-hamster kidney cells. Biochem J 276(2):349–355
Mitra A, Zhang Z (1994) Expression of a human lactoferrin cDNA in tobacco cells produces antibacterial protein (s). Plant Physiol 106(3):977–981
Min SR, Woo J, Jeong W, Han S, Lee Y, Liu JR (2006) Production of human lactoferrin in transgenic cell suspension cultures of sweet potato. Biol Plant 50:131–134
Cooper CA, Maga EA, Murray JD (2015) Production of human lactoferrin and lysozyme in the milk of transgenic dairy animals: past, present, and future. Transgenic Res 24:605–614
Goldman I, Georgieva S, Gurskiy YG, Krasnov A, Deykin A, Popov A, Ermolkevich T, Budzevich A, Chernousov A, Sadchikova E (2012) Production of human lactoferrin in animal milk. Biochem Cell Biol 90(3):513–519
Yemets AI, Tanasienko IV, Krasylenko YA, Blume YB (2014) Plant-based biopharming of recombinant human lactoferrin. Cell Biol Int 38(9):989–1002
Huang N, Bethell D, Card C, Cornish J, Marchbank T, Wyatt D, Mabery K, Playford R (2008) Bioactive recombinant human lactoferrin, derived from rice, stimulates mammalian cell growth. In Vitro Cell Dev Biol-Anim 44:464–471
Vega-Bautista A, de la Garza M, Carrero JC, Campos-Rodríguez R, Godínez-Victoria M, Drago-Serrano ME (2019) The impact of Lactoferrin on the growth of intestinal inhabitant bacteria. Int J Mol Sci 20(19):4707
Guo HY, Jiang L, Ibrahim SA, Zhang L, Zhang H, Zhang M, Ren FZ (2009) Orally administered lactoferrin preserves bone mass and microarchitecture in ovariectomized rats. J Nutr 139(5):958–964
Bharadwaj S, Naidu A, Betageri G, Prasadarao N, Naidu A (2009) Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporos Int 20:1603–1611
Cornish J, Naot D (2010) Lactoferrin as an effector molecule in the skeleton. Biometals 23:425–430
Georgieff MK (2008) The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans 36(6):1267–1271
Somm E, Larvaron P, Van De Looij Y, Toulotte A, Chatagner A, Faure M, Métairon S, Mansourian R, Raymond F, Gruetter R (2014) Protective effects of maternal nutritional supplementation with lactoferrin on growth and brain metabolism. Pediatr Res 75(1):51–61
Li W, Liu B, Lin Y, Xue P, Lu Y, Song S, Li Y, Szeto IM-Y, Ren F, Guo H (2022) The application of lactoferrin in infant formula: the past, present and future. Crit Rev Food Sci Nutr 1–20. https://doi.org/10.1080/10408398.2022.2157792
Li F, Wu SS, Berseth CL, Harris CL, Richards JD, Wampler JL, Zhuang W, Cleghorn G, Rudolph CD, Liu B (2019) Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: a randomized, controlled trial. J Pediatr 215:24-31.e8
King JC Jr, Cummings GE, Guo N, Trivedi L, Readmond BX, Keane V, Feigelman S, de Waard R (2007) A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J Pediatr Gastroenterol Nutr 44(2):245–251
Johnston WH, Ashley C, Yeiser M, Harris CL, Stolz SI, Wampler JL, Wittke A, Cooper TR (2015) Growth and tolerance of formula with lactoferrin in infants through one year of age: double-blind, randomized, controlled trial. BMC Pediatr 15(1):1–11
Chen K, Zhang G, Chen H, Cao Y, Dong X, Li H, Liu C (2020) Dose effect of bovine lactoferrin fortification on iron metabolism of anemic infants. J Nutr Sci Vitaminol 66(1):24–31
Aly E, López-Nicolás R, Darwish AA, Frontela-Saseta C, Ros-Berruezo G (2016) Supplementation of infant formulas with recombinant human lactoferrin and/or galactooligosaccharides increases iron bioaccessibility as measured by ferritin formed in Caco-2 cell model. Food Res Int 89:1048–1055
EFSA Panel on Dietetic Products N (2012) Allergies, scientific opinion on bovine lactoferrin. EFSA J 10(5):2701
Ochoa TJ, Zegarra J, Cam L, Llanos R, Pezo A, Cruz K, Zea-Vera A, Cárcamo C, Campos M, Bellomo S (2015) Randomized controlled trial of lactoferrin for prevention of sepsis in Peruvian neonates< 2500 grams. Pediatr Infect Dis J 34 (6):571
Alagawany M, Attia YA, Farag MR, Elnesr SS, Nagadi SA, Shafi ME, Khafaga AF, Ohran H, Alaqil AA, Abd El-Hack ME (2021) The strategy of boosting the immune system under the COVID-19 pandemic. Front Vet Science 7:570748
Ferrero-Miliani L, Nielsen O, Andersen P, Girardin S (2007) Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol 147(2):227–235
Saeed M, Alagawany M, Arain MA, Abd ME, El-Hack KD (2017) Beneficial impacts of choline in animal and human with special reference to its role against fatty liver syndrome. J Exp Biol 5:589–598
Russo M, Tedesco I, Iacomino G, Palumbo R, Galano G, Russo G (2005) Dietary phytochemicals in chemoprevention of cancer. Curr Med Chem-Immunol Endocr Metab Agents 5(1):61–72
Legrand D, Mazurier J (2010) A critical review of the roles of host lactoferrin in immunity. Biometals 23:365–376
Puddu P, Valenti P, Gessani S (2009) Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie 91(1):11–18
Siqueiros-Cendón T, Arévalo-Gallegos S, Iglesias-Figueroa BF, García-Montoya IA, Salazar-Martínez J, Rascón-Cruz Q (2014) Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin 35(5):557–566
Embleton ND, Berrington JE, McGuire W, Stewart CJ, Cummings SP (2013) In Lactoferrin: antimicrobial activity and therapeutic potential, Seminars in Fetal and Neonatal Medicine. Elsevier 2013:143–149
Kruzel M, Actor J, Boldogh I, Zimecki M (2007) Lactoferrin in health and disease. Adv Hyg Exp Med 61:261–267
Yen C-C, Shen C-J, Hsu W-H, Chang Y-H, Lin H-T, Chen H-L, Chen C-M (2011) Lactoferrin: an iron-binding antimicrobial protein against Escherichia coli infection. Biometals 24:585–594
Edde L, Hipolito RB, Hwang FF, Headon DR, Shalwitz RA, Sherman MP (2001) Lactoferrin protects neonatal rats from gut-related systemic infection. Am J Physiol-Gastrointest Liver Physiol 281(5):G1140–G1150
Mulder AM, Connellan PA, Oliver CJ, Morris CA, Stevenson LM (2008) Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr Res 28(9):583–589
Piacentini R, Centi L, Miotto M, Milanetti E, Di Rienzo L, Pitea M, Piazza P, Ruocco G, Boffi A, Parisi G (2022) Lactoferrin inhibition of the complex formation between ACE2 receptor and SARS CoV-2 recognition binding domain. Int J Mol Sci 23(10):5436
Badr H, Nabil NM, Tawakol MM (2021) Effects of the prebiotic lactoferrin on multidrug-resistant Escherichia coli infections in broiler chickens. Vet World 14(8):2197
Giardi MF, La Torre C, Giansanti F, Botti D (2009) Effects of transferrins and cytokines on nitric oxide production by an avian lymphoblastoid cell line infected with Marek’s disease virus. Antiviral Res 81(3):248–252
Hwang S-A, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, Kruzel ML, Actor JK (2007) Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine 25(37–38):6730–6743
Hung C-M, Yeh C-C, Chen H-L, Lai C-W, Kuo M-F, Yeh M-H, Lin W, Tu M-Y, Cheng H-C, Chen C-M (2010) Porcine lactoferrin administration enhances peripheral lymphocyte proliferation and assists infectious bursal disease vaccination in native chickens. Vaccine 28(16):2895–2902
Kawasaki Y, Sato K, Shinmoto H, Dosako SI (2000) Role of basic residues of human lactoferrin in the interaction with B lymphocytes. Biosci Biotechnol Biochem 64(2):314–318
Dhennin-Duthille I, Masson M, Damiens E, Fillebeen C, Spik G, Mazurier J (2000) Lactoferrin upregulates the expression of CD4 antigen through the stimulation of the mitogen-activated protein kinase in the human lymphoblastic T Jurkat cell line. J Cell Biochem 79(4):583–593
Legrand D, Elass E, Carpentier M, Mazurier J (2005) Lactoferrin: lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci 62:2549–2559
Hwang S-A, Kruzel ML, Actor JK (2005) Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int Immunopharmacol 5(3):591–599
Kruzel ML, Zimecki M, Actor JK (2017) Lactoferrin in a context of inflammation-induced pathology. Front Immunol 8:1438
Chen GY, Nuñez G (2010) Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10(12):826–837
Oppenheim JJ, Tewary P, de la Rosa G, Yang D (2007) Alarmins initiate host defense. Immune-Mediated Dis Theor Ther 601:185–194
De la Rosa G, Yang D, Tewary P, Varadhachary A, Oppenheim JJ (2008) Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol 180(10):6868–6876
Yang D, de la Rosa G, Tewary P, Oppenheim JJ (2009) Alarmins link neutrophils and dendritic cells. Trends Immunol 30(11):531–537
Welsh KJ, Hwang S-A, Boyd S, Kruzel ML, Hunter RL, Actor JK (2011) Influence of oral lactoferrin on Mycobacterium tuberculosis induced immunopathology. Tuberculosis 91:S105–S113
Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too? Curr Biol 27(14):R713–R715
Kruzel ML, Bacsi A, Choudhury B, Sur S, Boldogh I (2006) Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology 119(2):159–166
Madkour AH, Helal MG, Said E, Salem HA (2022) Dose-dependent renoprotective impact of Lactoferrin against glycerol-induced rhabdomyolysis and acute kidney injury. Life Sci 302:120646
Liu C, Peng Q, Wei L, Li Z, Zhang X, Wu Y, Wang J, Zheng X, Wen Y, Zheng R (2022) Deficiency of Lactoferrin aggravates lipopolysaccharide-induced acute inflammation via recruitment macrophage in mice. BioMetals 1–14. https://doi.org/10.1007/s10534-022-00398-1
Fan L, Yao Q, Wu H, Wen F, Wang J, Li H, Zheng N (2022) Protective effects of recombinant lactoferrin with different iron saturations on enteritis injury in young mice. J Dairy Sci 105(6):4791–4803
Marnett LJ (2000) Oxyradicals and DNA damage. Carcinogenesis 21(3):361–370
Misonou H, Morishima-Kawashima M, Ihara Y (2000) Oxidative stress induces intracellular accumulation of amyloid β-protein (Aβ) in human neuroblastoma cells. Biochemistry 39(23):6951–6959
Legrand D (2016) Overview of lactoferrin as a natural immune modulator. J Pediatr 173:S10–S15
Fillebeen C, Ruchoux M-M, Mitchell V, Vincent S, Benaı̈ssa M, Pierce A (2001) Lactoferrin is synthesized by activated microglia in the human substantia nigra and its synthesis by the human microglial CHME cell line is upregulated by tumor necrosis factor α or 1-methyl-4-phenylpyridinium treatment. Mol Brain Res 96(1–2):103–113
Safaeian L, Javanmard SH, Mollanoori Y, Dana N (2015) Cytoprotective and antioxidant effects of human lactoferrin against H2O2-induced oxidative stress in human umbilical vein endothelial cells. Adv Biomed Res 4:1–6
Safaeian L, Zabolian H (2014) Antioxidant effects of bovine lactoferrin on dexamethasone-induced hypertension in rat. Int Sch Res Not 2014:943523
Raychaudhuri R, Pandey A, Das S, Nannuri SH, Joseph A, George SD, Vincent AP, Mutalik S (2021) Nanoparticle impregnated self-supporting protein gel for enhanced reduction in oxidative stress: a molecular dynamics insight for lactoferrin-polyphenol interaction. Int J Biol Macromol 189:100–113
Arias M, Hilchie AL, Haney EF, Bolscher JG, Hyndman ME, Hancock RE, Vogel HJ (2017) Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem Cell Biol 95(1):91–98
Li H-Y, Li M, Luo C-C, Wang J-Q, Zheng N (2017) Lactoferrin exerts antitumor effects by inhibiting angiogenesis in a HT29 human colon tumor model. J Agric Food Chem 65(48):10464–10472
Li H, Yao Q, Min L, Huang S, Wu H, Yang H, Fan L, Wang J, Zheng N (2020) The combination of two bioactive constituents, lactoferrin and linolenic acid, inhibits mouse xenograft esophageal tumor growth by downregulating lithocholyltaurine and inhibiting the JAK2/STAT3-related pathway. ACS Omega 5(33):20755–20764
Stransky L, Cotter K, Forgac M (2016) The function of V-ATPases in cancer. Physiol Rev 96(3):1071–1091
Pereira CS, Guedes JP, Gonçalves M, Loureiro L, Castro L, Gerós H, Rodrigues LR, Côrte-Real M (2016) Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget 7(38):62144
Elzoghby AO, Abdelmoneem MA, Hassanin IA, Abd Elwakil MM, Elnaggar MA, Mokhtar S, Fang J-Y, Elkhodairy KA (2020) Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 263:120355
Gibbons JA, Kanwar JR, Kanwar RK (2015) Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer 15(1):1–16
Shankaranarayanan JS, Kanwar JR, Al-Juhaishi AJA, Kanwar RK (2016) Doxorubicin conjugated to immunomodulatory anticancer lactoferrin displays improved cytotoxicity overcoming prostate cancer chemo resistance and inhibits tumour development in TRAMP mice. Sci Rep 6(1):1–16
Zhang Z, Yang J, Min Q, Ling C, Maiti D, Xu J, Qin L, Yang K (2019) Holo-lactoferrin modified liposome for relieving tumor hypoxia and enhancing radiochemotherapy of cancer. Small 15(6):1803703
Jahani S, Shakiba A, Jahani L (2015) The Antimicrobial effect of lactoferrin on Gram-negative and Gram-positive bacteria. Int J Infect 2(3):e27954
Valenti P, Antonini G (2005) Lactoferrin: lactoferrin: an important host defence against microbial and viral attack. Cell Mol Life Sci 62:2576–2587
Baker EN, Baker HM (2009) A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 91(1):3–10
Ellison R 3rd, Giehl TJ, LaForce FM (1988) Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun 56(11):2774–2781
Kutila T, Pyörälä S, Saloniemi H, Kaartinen L (2003) Antibacterial effect of bovine lactoferrin against udder pathogens. Acta Vet Scand 44(1):1–8
Vongbhavit K, Underwood MA (2016) Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin Ther 38(4):716–732
Acosta-Smith E, Viveros-Jimenez K, Canizalez-Roman A, Reyes-Lopez M, Bolscher JG, Nazmi K, Flores-Villasenor H, Alapizco-Castro G, De la Garza M, Martínez-Garcia JJ (2018) Bovine lactoferrin and lactoferrin-derived peptides inhibit the growth of Vibrio cholerae and other Vibrio species. Front Microbiol 8:2633
León-Sicairos N, Angulo-Zamudio UA, Vidal JE, López-Torres CA, Bolscher JG, Nazmi K, Reyes-Cortes R, Reyes-López M, de la Garza M, Canizalez-Román A (2014) Bactericidal effect of bovine lactoferrin and synthetic peptide lactoferrin chimera in Streptococcus pneumoniae and the decrease in lux S gene expression by lactoferrin. Biometals 27:969–980
Bellés A, Aguirre-Ramírez D, Abad I, Parras-Moltó M, Sánchez L, Grasa L (2022) Lactoferrin modulates gut microbiota and Toll-like receptors (TLRs) in mice with dysbiosis induced by antibiotics. Food Funct 13(10):5854–5869
Al-Nabulsi A, Holley R (2006) Enhancing the antimicrobial effects of bovine lactoferrin against Escherichia coli O157: H7 by cation chelation, NaCl and temperature. J Appl Microbiol 100(2):244–255
André G, Politano W, Mirza S, Converso T, Ferraz L, Leite L, Darrieux M (2015) Combined effects of lactoferrin and lysozyme on Streptococcus pneumoniae killing. Microb Pathog 89:7–17
Hwang S-A, Kruzel ML, Actor JK (2014) Immunomodulatory effects of recombinant lactoferrin during MRSA infection. Int Immunopharmacol 20(1):157–163
Redwan EM, Abd El-Baky N, Al-Hejin AM, Baeshen MN, Almehdar HA, Elsaway A, Gomaa A-BM, Al-Masaudi SB, Al-Fassi FA, AbuZeid IE (2016) Significant antibacterial activity and synergistic effects of camel lactoferrin with antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). Res Microbiol 167(6):480–491
Fukuta S, Kawamoto K-I, Mizukami Y, Yoshimura Y, Ueda J-I, Kanbe M (2012) Transgenic tobacco plants expressing antimicrobial peptide bovine lactoferricin show enhanced resistance to phytopathogens. Plant Biotechnol 29(4):383–389
Duarte LG, Alencar WM, Iacuzio R, Silva NC, Picone CS (2022) Synthesis, characterization and application of antibacterial lactoferrin nanoparticles. Curr Res Food Sci 5:642–652
Kieckens E, Rybarczyk J, Cox E, Vanrompay D (2018) Antibacterial and immunomodulatory activities of bovine lactoferrin against Escherichia coli O157: H7 infections in cattle. Biometals 31:321–330
Yen C-C, Lim C-Y, Chong K-Y, Tsai T-C, Shen C-J, Lim M-F, Su C-Y, Chen H-L, Chen C-M (2009) Lactoferrin as a natural regimen for selective decontamination of the digestive tract: recombinant porcine lactoferrin expressed in the milk of transgenic mice protects neonates from pathogenic challenge in the gastrointestinal tract. J Infect Dis 199(4):590–598
Tanhaiean A, Azghandi M, Razmyar J, Mohammadi E, Sekhavati MH (2018) Recombinant production of a chimeric antimicrobial peptide in E. coli and assessment of its activity against some avian clinically isolated pathogens. Microb Pathog 122:73–78
Jean C, Boulianne M, Britten M, Robitaille G (2016) Antimicrobial activity of buttermilk and lactoferrin peptide extracts on poultry pathogens. J Dairy Res 83(4):497–504
Tang X-S, Tang Z-R, Wang S-P, Feng Z-M, Zhou D, Li T-J, Yin Y-L (2012) Expression, purification, and antibacterial activity of bovine lactoferrampin–lactoferricin in Pichia pastoris. Appl Biochem Biotechnol 166:640–651
Yin C, Wong J, Ng T (2014) Recent studies on the antimicrobial peptides lactoferricin and lactoferrampin. Curr Mol Med 14(9):1139–1154
Beeckman D, Van Droogenbroeck C, De Cock B, Van Oostveldt P, Vanrompay D (2007) Effect of ovotransferrin and lactoferrins on Chlamydophila psittaci adhesion and invasion in HD11 chicken macrophages. Vet Res 38(5):729–739
Ward P, Paz E, Conneely O (2005) Lactoferrin: multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci 62:2540–2548
Kanwar J, Samarasinghe R, Sehgal R, Kanwar R (2012) Nano-lactoferrin in diagnostic, imaging and targeted delivery for cancer and infectious diseases. Cancer Sci Ther 4(3):31–42
Kanwar KR, Singh N, Gurudevan S, Kanwar RJ (2011) Targeting hepatitis B virus and human papillomavirus induced carcinogenesis: novel patented therapeutics. Recent Patents Anti-Infect Drug Discov 6(2):158–174
Chen H-L, Wang L-C, Chang C-H, Yen C-C, Cheng WT, Wu S-C, Hung C-M, Kuo M-F, Chen C-M (2008) Recombinant porcine lactoferrin expressed in the milk of transgenic mice protects neonatal mice from a lethal challenge with enterovirus type 71. Vaccine 26(7):891–898
Redwan EM, Uversky VN, El-Fakharany EM, Al-Mehdar H (2014) Potential lactoferrin activity against pathogenic viruses. CR Biol 337(10):581–595
Puddu P, Borghi P, Gessani S, Valenti P, Belardelli F, Seganti L (1998) Antiviral effect of bovine lactoferrin saturated with metal ions on early steps of human immunodeficiency virus type 1 infection. Int J Biochem Cell Biol 30(9):1055–1063
Belting M (2003) Heparan sulfate proteoglycan as a plasma membrane carrier. Trends Biochem Sci 28(3):145–151
Pietrantoni A, Dofrelli E, Tinari A, Ammendolia MG, Puzelli S, Fabiani C, Donatelli I, Superti F (2010) Bovine lactoferrin inhibits influenza A virus induced programmed cell death in vitro. Biometals 23:465–475
Pietrantoni A, Di Biase AM, Tinari A, Marchetti M, Valenti P, Seganti L, Superti F (2003) Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob Agents Chemother 47(8):2688–2691
Kaito M, Iwasa M, Fujita N, Kobayashi Y, Kojima Y, Ikoma J, Imoto I, Adachi Y, Hamano H, Yamauchi K (2007) Effect of lactoferrin in patients with chronic hepatitis C: combination therapy with interferon and ribavirin. J Gastroenterol Hepatol 22(11):1894–1897
Li S, Zhou H, Huang G, Liu N (2009) Inhibition of HBV infection by bovine lactoferrin and iron-, zinc-saturated lactoferrin. Med Microbiol Immunol 198:19–25
Campione E, Lanna C, Cosio T, Rosa L, Conte MP, Iacovelli F, Romeo A, Falconi M, Del Vecchio C, Franchin E (2021) Lactoferrin against SARS-CoV-2: in vitro and in silico evidences. Front Pharmacol 12:666600
Salaris C, Scarpa M, Elli M, Bertolini A, Guglielmetti S, Pregliasco F, Blandizzi C, Brun P, Castagliuolo I (2021) Protective effects of lactoferrin against SARS-CoV-2 infection in vitro. Nutrients 13(2):328
Mirabelli C, Wotring JW, Zhang CJ, McCarty SM, Fursmidt R, Pretto CD, Qiao Y, Zhang Y, Frum T, Kadambi NS (2021) Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19. Proceed Natl Acad Sci 118(36):e2105815118
Author information
Authors and Affiliations
Contributions
All authors were equal contributors in writing this review article.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashraf, M.F., Zubair, D., Bashir, M.N. et al. Nutraceutical and Health-Promoting Potential of Lactoferrin, an Iron-Binding Protein in Human and Animal: Current Knowledge. Biol Trace Elem Res 202, 56–72 (2024). https://doi.org/10.1007/s12011-023-03658-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03658-4