Abstract
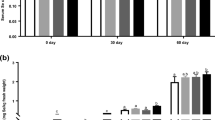
Selenium (Se) is an essential element and antioxidant that catalyzes the destruction of hydrogen peroxide formed during cellular oxidative metabolism. Doses of Se as selenomethionine (SeMe) by oral route are 0.1–0.3 mgSe/kg DM, while the dose by parenteral route with sodium selenite (Na2SeO3) is 0.1 mgSe/BW. The effects of supranutritional Se supplementation on normal kids have rarely been studied. The objective of the study was to evaluate both Se sources on growth performance, Se in tissues, histopathological findings, and meat characteristics. Forty-five kids of the Pastoreña breed with 25-day age were distributed (4.7 ± 1.13 kg) in three treatments: a) control group, C: consumption with goat milk (GM: containing 0.135 mgSe/g); b) NaSe: GM plus Na2SeO3 injectable, 0.25 mgSe/kg BW; c) SeMe: GM plus oral dosage, 0.3 mgSe as SeMe daily. Fifteen animals per treatment were slaughtered at 7, 14, and 21 days. Feed conversion improved (P < 0.05) with Se supplement (P < 0.05) at 7 and 14 days. SeMe had higher protein and fat meat content (P < 0.05). SeMe increased Se liver at 14 and 21 days. NaSe and SeMe had higher (P < 0.05) levels of Se kidney. SeMe-21d showed 42% mononuclear and periportal cell infiltration lesions. In conclusion, Se administered through milk in goat kids was insufficient to prevent nutritional muscular dystrophy. The supranutritional dose of 0.25 mg/kg as NaSe was sufficient to maintain the Se level in tissues. SeMe increased Se liver and kidney efficiently. Both Se sources improved the bioavailability of the mineral in kids.



Similar content being viewed by others
References
Gupta UC, Gupta SC (2000) Selenium in soils and crops, its deficiencies in livestock and humans : implications for management. Commun Soil Sci Plant Anal 31(11–14):1791–1807
Yumusak N, Yigin A, Polat PF, Hitit M, Yilmaz R (2018) Expression of ADAMTS-7 in myocardial dystrophy associated with white muscle disease in lambs. Pol J Vet Sci 21(1):119–126. https://doi.org/10.24425/119029
Mccomb T, Bischoff K, Thompson B, Smith MC, Mohammed HO, Ebel J, Hillebrandt J (2010) An investigation of bloodselenium concentrations of goats in New York state. J Vet Diagn Invest 22(5):696–701. https://doi.org/10.1177/104063871002200504
Sheppard AD, Blom L, Grant AB (2011) Levels of selenium in blood and tissues associated with some selenium deficiency diseases in New Zealand sheep. N Z Vet J 32(6):91–95. https://doi.org/10.1080/00480169.1984.35076
Youde H (2002) An experimental study on the treatment and prevention of Shimao zheng (fleece-eating) in sheep and goats in the Haizi area of Akesai county in China. Vet Res Commun 26(1):39–48
Koller LD, Exon JH (1986) The two faces of selenium - deficiency and toxicity - are similar in animals and man. Can J Vet Res 50(3):297–306
NRC (2007) Nutrient requirements of small ruminants: sheep, goats, cervids, and New World Camelids, 7th edn. Animal nutrition series. National Academy Press, Washington (DC), pp 134–137. https://doi.org/10.17226/11654
Samo SP, Malhi M, Kachiwal AB, Gadahi JA, Parveen F (2020) Supranutritional selenium level minimizes high concentrate diet-induced epithelial injury by alleviating oxidative stress and apoptosis in colon of goat. BMC Vet Res 16(462):1–10
Taylor JB, Marchello MJ, Finley JW, Neville TL, Combs GCJ (2008) Nutritive value and display-life attributes of selenium-enriched beef-muscle foods. J Food Compos Anal 21:183–186
Spears JW (2003) Comparative trace element nutrition trace mineral bioavailability in ruminants. J Nutr [Internet] 133(11):1506S–1509S. Available from: https://academic.oup.com/jn/article/133/5/1506S/4558538. Accessed 5 Jan 2022
Surai PF, Fisinin VI (2016) Selenium in sow nutrition. Anim Feed Sci Technol 211:18–30
Mahan DC, Cline TR, Richert B (1999) Effects of dietary levels of selenium-enriched yeast and sodium selenite as selenium sources fed to growing-finishing pigs on performance, tissue selenium, serum glutathione peroxidase activity, carcass characteristics, and loin quality. J Anim Sci 77(8):2172–2179
Ortman KP, B, (1999) Effect of selenate as a feed supplement to dairy cows in comparison to selenite and selenium yeast. J Anim Sci 77(12):3365–3370
Osman ML, J, (1976) Biological potency of selenium from sodium selenite, selenomethionine and selenocystine in the chick. Poult Sci 55:987–994
Xing H, Zheng S, Zhang Z, Zhu F, Xue H, Xu S (2019) Pharmacokinetics of selenium in healthy piglets after different routes of administration: application of pharmacokinetic data to the risk assessment of selenium. Biol Trace Elem Res 191(2):403–411
SEMARNAT (1999) Especificaciones técnicas para la producción, cuidado y uso de animales de laboratorio. NORMA Oficial Mexicana NOM-062-ZOO 2011:2
Dficial D (2015) (2014) NORMA Oficial Mexicana NOM-033-SAG/ZOO. Métodos para dar muerte a los animales domésticos y silvestres. D Of la Fed 7(2):44–68
Bezerra SBL, Véras ASC, de Andrade Silva DK, de Andrade FM, Pereira KP, de Arruda Santos GR, Rodrigues AL, Cavalcanti AM (2012) Morphometry and carcass characteristics of goats submitted to grazing in the Caatinga. Rev Bras Zootec 41(1):131–137
Colomer-Rocher F, Morand-Fehr P (1987) Standard methods and procedures for goat carcass evaluation, jointing and tissue separation. Livest Prod Sci [Internet] 17:149–59. https://doi.org/10.1016/0301-6226(87)90060-1
AOAC (2005) Official methods of analysis of AOAC international, 18th edn. In: Lee J, Durst R, Wrolstad R (eds). Washington, DC
SAS (2016) User’s guide. Cary, NC USA, p 464
Pechová A, Antošová L, Pavlata L, Podhorský A (2015) Effect of sodium selenite or lactate-protein selenium complex supplementation on selenium status in goat kids. Czech J Anim Sci 60(1):16–24
Tian X-Z, Li J-X, Luo Q-Y, Wang X, Xiao M-M, Zhou D, Lu Q, Chen X (2022) Effect of supplementation with selenium-yeast on muscle antioxidant activity, meat quality, fatty acids and amino acids in goats. Front Vet Sci 8:813672. https://doi.org/10.3389/fvets.2021.813672
Chauhan SS, Ponnampalam EN, Celi P, Hopkins DL, Leury BJ, Dunshea FR (2016) High dietary vitamin E and selenium improves feed intake and weight gain of finisher lambs and maintains redox homeostasis under hot conditions. Small Rumin Res [Internet] 137:17–23. https://doi.org/10.1016/j.smallrumres.2016.02.011
Samo S, Malhi M, Gadahi J, Lei Y, Kaciwal AB, Soomro SA (2018) Effect of organic selenium supplementation in diet on gastrointestinal tract performance. Pakistan J Zool 50(3):995–1003
Pérez P, Maino M, Morales MS, Soto A (2001) Effect of goat milk and milk substitutes and sex on productive parameters and carcass composition of Creole kids. Small Rumin Res [Internet] 42(1):87–94. https://doi.org/10.1016/S0921-4488(01)00233-4
De Palo P, Maggiolino A, Centoducati N, Tateo A (2015) Effects of different milk replacers on carcass traits, meat quality, meat color and fatty acids profile of dairy goat kids. Small Rumin Res [Internet] 131:6–11. https://doi.org/10.1016/j.smallrumres.2015.09.001
Pophiwa P, Webb EC, Frylinck L (2017) Carcass and meat quality of Boer and indigenous goats of South Africa under delayed chilling conditions. S Afr J Anim Sci 47(6):794–803
Shija DS, Mtenga LA, Kimambo AE, Laswai GH, Mushi DE, Mgheni DM, Mwilawa AJ, Shirima EJ, Safari JG (2013) Chemical composition and meat quality attributes of indigenous sheep and goats from traditional production system in Tanzania. Asian-Australasian J Anim Sci [Internet] 26(2):295–302. https://doi.org/10.5713/ajas.2012.12432
Vignola G, Lambertini L, Mazzone G, Giammarco M, Tassinari M, Martelli G, Bertin G (2009) Effects of selenium source and level of supplementation on the performance and meat quality of lambs. Meat Sci 81:678–685. https://doi.org/10.1016/j.meatsci.2008.11.009
Sgoifo CA, Compiani R, Baldi G, Bernardi C, Muraro M, Marden J-P, Dell’Orto V (2015) The effect of different selenium sources during the finishing phase on beef quality. J Anim Feed Sci 24:93–99. https://doi.org/10.3390/antiox10040596
Grossi S, Rossi L, De Marco M, Sgoifo CA (2021) The effect of different sources of selenium supplementation on the meat quality traits of young Charolais bulls during the finishing phase. Antioxidants 10:596. https://doi.org/10.3390/antiox10040596
Zurita-Herrera P, Delgado JV, Argüello A, Camacho ME (2011) Multivariate analysis of meat production traits in Murciano-Granadina goat kids. Meat Sci [Internet] 88(3):447–453. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0309174011000386. Accessed 10 Feb 2022
Das AK, Rajkumar V (2010) Comparative study on carcass characteristics and meat quality of. Indian J Anim Sci 80(10):1014–1018
Freitas HS, Alcalde CR, De LLS, De F, De MAF, Macedo VDP, Molina BS (2011) Quantitative characteristics of carcass and meat quality of ¾ Boer + ¼ Saanen and Saanen goat kids fed diets with dry yeast. Rev Bras Zootec 40(3):630–638
Lagerstedt Å, Enfält L, Johansson L, Lundström K (2008) Effect of freezing on sensory quality, shear force and water loss in beef M. longissimus dorsi. Meat Sci. 80(2):457–61
Martínez MAL (2008) Nutrición y calidad de la carne de los rumiantes - Nutrition and quality of meat from ruminant animals. Rev Electron Vet 9(10):1–21
Li M, Zhang Y, Li S (2020) Effects of selenium deficiency on testis development and autophagy in chicks. Ital J Anim Sci [Internet] 19(1):753–61. https://doi.org/10.1080/1828051X.2020.1786739
Şen U, Şirin E, Filik G. Soidan, E, (2020) The effect of breed on instrumental meat quality traits of weaning kids from Turkish indigenous goat breeds Breed effect on kid meat quality. Large Anim Rev 26:19–24
Ivanović S, Pavlović M, Pavlović I, Tasić A, Janjić J, Baltić MZ (2020) Influence of breed on selected quality parameters of fresh goat meat. Arch Anim Breed [Internet]. 63:219–29. https://doi.org/10.5194/aab-63-219-2020
Kawęcka A, Sikora J, Gąsior R, Puchała M, Wojtycza K (2022) Comparison of carcass and meat quality traits of the native Polish heath lambs and the Carpathian kids. J Appl Anim Res 50(1):109–117
Milagro FI, Campión J, Martíez JA (2006) Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity 14(7):1118–1123
Santos NL, De SWH, Cunha G, Sancha A (2020) Meat quality of suckling goat raised in differents feeding systems. Anim Prod 42:1–7
Purslow PP, Gagaoua M, Warner RD (2021) Insights on meat quality from combining traditional studies and proteomics. Meat Sci [Internet] 174:108423. https://doi.org/10.1016/j.meatsci.2020.108423
Sheridan R, Hoffman LC, Ferreira AV (2003) Meat quality of Boer goat kids and Mutton Merino lambs 2. Sensory meat evaluation. Anim Sci 76(1):73–79
Ryan SM, Unruh JA, Corrigan ME, Drouillard JS, Seyfert M (2007) Effects of concentrate level on carcass traits of Boer crossbred goats. Small Rumin Res 73:67–76. https://doi.org/10.1016/j.smallrumres.2006.11.004
Daun C, Johansson M, Önning G, Åkesson B (2001) Glutathione peroxidase activity, tissue and soluble selenium content in beef and pork in relation to meat ageing and pig RN phenotype. Food Chem 73:313–319. https://doi.org/10.1016/s0308-8146(00)00303-4
Teixeira A, Jimenez-Badillo MR, Rodrigues S (2011) Effect of sex and carcass weight oh carcass traits and meat quality in goat kids of Cabrito Transmontano. Spanish J Agric Res 9(3):7753–7760
Martínez LC, Hernández-Bautista JR de la CLC (2011) Rendimiento de la canal y características físico-químicas de la carde de cabritos sacrificados a diferente peso vivo. In: Nacameh [Internet], p 38–39. Available from: http://cbs.izt.uam.mx/nacameh/. Accessed 11 Feb 2022
Bañón S, Vila R, Price A, Ferrandini E, Garrido MD (2006) Effects of goat milk or milk replacer diet on meat quality and fat composition of suckling goat kids. Meat Sci [Internet] 72(2):216–21. https://doi.org/10.1016/j.meatsci.2005.07.004
Hopkins DL, Ponnampalam EN, van de Ven RJ, Warner RD (2014) The effect of pH decline rate on the meat and eating quality of beef carcasses. Anim Prod Sci 54:407–413. https://doi.org/10.1071/AN12314
Ramirez-Bribiesca JE, Tortora JL, Huerta M, Aguirre A, Hernandez LM (2001) Diagnosis of selenium status in grazing dairy goats on the Mexican plateau. Small Rumin Res [Internet]. 41:81–5. https://doi.org/10.1016/S0921-4488(01)00188-2
Rodríguez Latorre CA, Flores Olivares CA, Luján Fernández E (2021) Determinación de selenio en caprinos de la localidad de Peldehue, en la región metropolitana de Santiago de Chile. Rev Med Vet (Bogota) 1(42):51–57
Sobiech P, Żarczyńska K (2020) The influence of selenium deficiency on chosen biochemical parameters and histopathological changes in muscles of goat kids. Pol J Vet Sci 23(2):267–279
Swanson CA, Patterson BH, Levander OA, Veillon C, Taylor PR, Helzlsouer K, McAdam PA, Zech LA (1991) Human [74Se]selenomethionine metabolism: a kinetic model. Am J Clin Nutr 54(5):917–926. https://doi.org/10.1093/ajcn/54.5.917
Rodriguez EMR, Alaejos MS, Romero CD (2002) Mineral content in goat’s milk. J Food Qual 25:343–358. https://doi.org/10.1111/j.1745.2002.tb01030.x
Raynal-Ljutovac K, Lagroffoul C, Paccard P, Guillet I, Chilliard Y (2008) Composition of goat and sheep milk products: an update. Small Rumin Res 79:57–72. https://doi.org/10.1016/j.smallrumres.2008.07.009
Sun L, Liu G, Xu D, Wu Z, Ma L, Sanz-Fernandez L, Victoria M, Baumgard LH, Dengpan B (2021) Milk selenium content and speciation in response to supranutritional selenium yeast supplementation in cows. Anim Nutr 7:1087–1094. https://doi.org/10.1016/j.aninu.2021.07.006
Tiwary AK, Stegelmeier BL, Panter KE, James LF, Hall JO (2006) Comparative toxicosis of sodium selenite and selenomethionine in lambs. J Vet Diagnostic Investig 18(1):61–70
Rammell CG, Thompson KG, Bentley GR, Gibbons MW (1989) Selenium, vitamin E and polyunsaturated fatty acid concentrations in goat kids with and without nutritional myodegeneration. N Z Vet J 37(1):4–6
Vorobyov V, Vorobyov D, Polkovnichenko P, Safonov V (2019) Evaluation of hematological and metabolic parameters in small ruminants with trace elements deficiency under different biogeochemical conditions. World’s Vet J 9(4):311–316
El G-H, López-Arellano R, Revilla-Vázquez A, Ramírez-Bribiesca E, Tórtora-Pérez J (2007) The relationship between fetal and maternal selenium concentrations in sheep and goats. Small Rumin Res 73(1–3):174–180
Suttle NF (2010) Mineral nutrition of livestock, 4th edn. CABI, UK, p 588
Zhang L, Liu XR, Liu JZ, An XP, Zhou ZQ, Cao BY, Song YX (2018) Supplemented organic and inorganic selenium affects milk performance and selenium concentration in milk and tissues in the Guanzhong dairy goat. Biol Trace Elem Res 183(2):254–260. https://doi.org/10.1007/s12011-017-1112-1
Rammell CG, Thompson KG, Bentley GR, Gibbons M (1989) Selenium, vitamin E and polyunsaturated fatty acid concentrations in goat kids with and without nutritional myodegeneration. N Z Vet J 37(1):4–6. https://doi.org/10.1080/00480169.1989.35536
Vorobyov V, Vorobyov D, Polkovnichenko P, Safonov V (2019) Evaluation of hematological and metabolic parameters in small ruminants with trace elements deficiency under different biogeochemical conditions. World Vet J 9(4):311–316. https://doi.org/10.36380/scil.2019.wvj39
Lopez-Arellano R, Ramirez-Bribiesca JE, Jaimes-Miranda J, Tortora-Perez JL, Revilla-Vazquez AL, Rodriguez-Patiño G, Montaño M (2015) Pathophysiological response to experimental oral overdose of different forms of selenium in lambs. Ann Anim Sci 15(3):655–666. https://doi.org/10.1515/aoas-2015-0016
Micheloud JF, Araoz V, Delgado FO, Colque-caro LA, Rosa DE, Mattioli GA (2018) Distrofia muscular nutricional en corderos de raza Dorper en el Noroeste Argentino. Rev Med Vet (B Aires) 99(2):13–16
Funding
This study was study was supported by the Colegio de Postgraduados Mexico and by the Scholarship of Consejo Nacional de Ciencia y Tecnología (CONACyT), México. The first author, Oscar Ortiz Morales (No. 283569), is a PhD student of the Program of Genetic Resources and Productivity–Livestock at the Graduate College (PREGEP-COLPOS).
Author information
Authors and Affiliations
Contributions
All the authors have read and approved the submission of this manuscript. Ortiz-Morales, Ramírez-Bribiesca, Hernández-Bautista, and Díaz-Sánchez contributed to the study concept and design. Ortiz-Morales, Barcena-Gama, Diaz-Sánchez, and Garrido-Fariña reached the animals. Ortiz-Morales, Hernández-Rodriguez, Hernández-Trujillo, and López-Ojeda were responsible for the biochemical analysis. Ortiz-Morales and Hernández-Sánchez did the statistical analyses. Ortiz-Morales and Ramírez-Bribiesca drafted the manuscript and interpreted the data. Hernández-Bautista, Bárcena-Gama, Díaz-Sánchez, and Hernández-Sánchez contributed to the critical revisions of the manuscript for important intellectual content. All the authors provided study supervision.
Corresponding author
Ethics declarations
Ethics approval
The ethic committee of the Colegio de Postgraduados approved all the procedures with the animals.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ortiz-Morales, O., Ramírez-Bribiesca, J.E., Hernández-Bautista, J. et al. Effect of Supranutritional Dosage Selenium in Neonatal Goat Kids on Productive Performance, Physicochemical Profiles in Meat, Selenium Levels in Tissues, and Histopathological Findings. Biol Trace Elem Res 201, 4374–4388 (2023). https://doi.org/10.1007/s12011-022-03528-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03528-5