Abstract
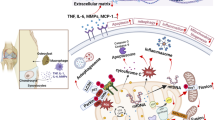
Selenoprotein S (SelS), a member of the selenoprotein family, is mainly located on the endoplasmic reticulum (ER) membrane. SelS is involved in a variety of biological processes, including oxidative stress, inflammation, glucose metabolism regulation, and ER-associated protein degradation (ERAD). This study was designed to explore the role of SelS in chondrocytes. It was confirmed that SelS is a Se-sensitive selenoprotein in low-selenium rat and cell models. ER stress was not induced in SelS knockdown ATDC5 cells. However, treatment of ATDC5 cells with tunicamycin (Tm), an ER stress inducer, increased the expression of SelS, and knockdown of SelS aggravated ER stress induced by Tm, suggesting that SelS is a regulatory molecule involved in ER stress in chondrocytes. Both osteoarthritis and Kashin-Beck disease are osteochondral diseases associated with hypertrophic chondrocyte abnormalities. Therefore, ATDC5 cells were induced to hypertrophic chondrocytes. SelS was knocked down and RNA sequencing was performed. Bioinformatics analysis of the differentially expressed genes (DEGs) revealed that SelS knockdown affected a variety of biological processes, including cell adhesion, osteoclast differentiation, and extracellular matrix homeostasis. Collectively, this study verified that SelS is sensitive to selenium levels and is an ER stress-responsive molecule. Knocking down SelS can cause abnormal expression of adhesion molecules and matrix homeostasis disorder in hypertrophic chondrocytes.





Similar content being viewed by others
References
Labunskyy VM, Hatfield DL, Gladyshev VN (2014) Selenoproteins: molecular pathways and physiological roles. Physiol Rev 94(3):739–777. https://doi.org/10.1152/physrev.00039.2013
Radomska D, Czarnomysy R, Radomski D, Bielawska A, Bielawski K (2021) Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 13(5):1649. https://doi.org/10.3390/nu13051649
Kipp AP, Strohm D, Brigelius-Flohé R, Schomburg L, Bechthold A, Leschik-Bonnet E, Heseker H (2015) Revised reference values for selenium intake. J Trace Elem Med Biol 32:195–199. https://doi.org/10.1016/j.jtemb.2015.07.005
Avery JC, Hoffmann PR (2018) Selenium, Selenoproteins, and Immunity. Nutrients 10(9):1203. https://doi.org/10.3390/nu10091203
Yan C, Luo R, Li F, Liu M, Li J, Hua W, Li X (2021) The epidemiological status, environmental and genetic factors in the etiology of Keshan disease. Cardiovasc Endocrinol Metab 10(1):14–21. https://doi.org/10.1097/XCE.0000000000000214
Lei Y, Zhao G, Fangfang Yu, Zhang R, Guo X (2016) Selenium and Iodine Levels in Subjects with Kashin-Beck Disease: a Meta-analysis. Biol Trace Elem Res 170(1):43–54. https://doi.org/10.1007/s12011-015-0463-8
Guo X, Ma WJ, Zhang F, Ren FL, Qu CJ, Lammi MJ (2014) Recent advances in the research of an endemic osteochondropathy in China: Kashin-Beck disease. Osteoarthritis Cartilage 22(11):1774–1783. https://doi.org/10.1016/j.joca.2014.07.023
Ren FL, Guo X, Zhang RJ, Geng D, Yu Y, Su M (2007) Effects of selenium and iodine deficiency on bone, cartilage growth plate and chondrocyte differentiation in two generations of rats. Osteoarthritis Cartilage 15(10):1171–1177. https://doi.org/10.1016/j.joca.2007.03.013
Kang D, Lee J, Wu CY, Guo X, Lee BJ, Chun JS, Kim JH (2020) The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp Mol Med 52(8):1198–1208. https://doi.org/10.1038/s12276-020-0408-y
Walder K, Kantham L, McMillan JS, Trevaskis J, Kerr L, de Silva A, Sunderland T, Godde N, Gao Y, Bishara N, Windmill K, Tenne-Brown J, Augert G, Zimme PZ, Collier GR (2002) Tanis: A Link Between Type 2 Diabetes and Inflammation? Diabetes 51(6):1859–1866. https://doi.org/10.2337/diabetes.51.6.1859
Shchedrina VA, Zhang Y, Labunskyy VM, Hatfield DL, Gladyshev VN (2010) Structure-function relations, physiological roles, and evolution of mammalian ER-resident selenoproteins. Antioxid Redox Signal 12(7):839–849. https://doi.org/10.1089/ars.2009.2865
Lee JH, Kwon JH, Jeon YH, Ko KY, Lee SR, Kim IY (2014) Pro178 and Pro183 of selenoprotein S are essential residues for interaction with p97(VCP) during endoplasmic reticulum-associated degradation. J Biol Chem 289(20):13758–13768. https://doi.org/10.1074/jbc.M113.534529
Lilley BN, Ploegh HL (2005) Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. PNAS 102(40):14296–14301. https://doi.org/10.1073/pnas.0505014102
Curran JE, Jowett M, JB, Elliott KS, Gao Y, Gluschenko K, Wang JM, Abel Azim DM, Cai GW, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, MacCluer JW, Collier GR, Kissebah AH, Blangero J, (2005) Genetic variation in selenoprotein S influences inflammatory response. Nat Genet 37(11):1234–1241. https://doi.org/10.1038/ng1655
Zhao Y, Li H, Men LL, Huang RC, Zhou HC, Xing Q, Yao JJ, Shi CH, Du JL (2013) Effects of selenoprotein S on oxidative injury in human endothelial cells. J Transl Med 11:287. https://doi.org/10.1186/1479-5876-11-287
Du XA, Wang HM, Dai XX, Cao JL, Mo YM, Xiong YM (2015) Role of selenoprotein S (SEPS1) -105G>A polymorphisms and PI3K/Akt signaling pathway in Kashin-Beck disease. Osteoarthritis Cartilage 23(2):210–216. https://doi.org/10.1016/j.joca.2014.11.017
He Y, Fan LH, Aaron N, Feng YP, Fang Q, Zhang Y, Zhang D, Wang H, Ma TY, Sun J, Chen JH (2022) Reduction of Smad2 caused by oxidative stress leads to necrotic death of hypertrophic chondrocytes associated with an endemic osteoarthritis. Rheumatology (Oxford) 61(1):440–451. https://doi.org/10.1093/rheumatology/keab286
Reeves PG, Nielsen FH, Fahey Jr GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutrition 123(11):1939–1951. https://doi.org/10.1093/jn/123.11.1939
Zhou XR, Yang HJ, Guan F, Xue SH, Song DQ, Chen JH, Wang ZL (2016) T-2 Toxin Alters the Levels of Collagen II and Its Regulatory Enzymes MMPs/TIMP-1 in a Low-Selenium Rat Model of Kashin-Beck Disease. Biol Trace Elem Res 169:237–246. https://doi.org/10.1007/s12011-015-0408-2
Yan JD, Zheng YW, Min ZX, Ning QL, Lu SM (2013) Selenium effect on selenoprotein transcriptome in chondrocytes. Biometals 26(2):285–296. https://doi.org/10.1007/s10534-013-9610-x
Liang WN, Li XH, Hu L, Ding SS, Kang J, Shen JY, Zheng CS, Li CD, Ye HZ, Asakawa T (2019) An in vitro validation of the therapeutic effects of Tougu Xiaotong capsule on tunicamycin-treated chondrocytes. J Ethnopharmacol 229:215–221. https://doi.org/10.1016/j.jep.2018.10.022
Lin PD, Weng XP, Liu FY, Ma YH, Chen HH, Shao X, Zheng WW, Liu XX, Ye HZ, Li XH (2015) Bushen Zhuangjin decoction inhibits TM-induced chondrocyte apoptosis mediated by endoplasmic reticulum stress. Int J Mol Med 36(6):1519–1528. https://doi.org/10.3892/ijmm.2015.2387
Rayman MP (2012) Selenium and human health. The Lancet 379(9822):1256–1268. https://doi.org/10.1016/S0140-6736(11)61452-9
Wang Q, Zhan S, Liu YQ, Han F, Shi LL, Han Chao MuWP, Cheng JZ, Huang ZW (2021) Low-Se Diet Can Affect Sperm Quality and Testicular Glutathione Peroxidase-4 activity in Rats. Biol Trace Elem Res 199(10):3752–3758. https://doi.org/10.1007/s12011-020-02515-y
Jia Y, Dai J, Zeng Z (2020) Potential relationship between the selenoproteome and cancer. Mol Clin Oncol 13(6):83. https://doi.org/10.3892/mco.2020.2153
Long ZD, Xiang JQ, Song JP, Lu YP, Yin HQ, Zhu YF, Liu XD, Qin LQ, Bañuelos GS, Wang ZM, Kang Y, Yuan LX, Yin XB (2020) Soil Selenium Concentration and Residents Daily Dietary Intake in a Selenosis Area: A Preliminary Study in Yutangba Village, Enshi City. China Bull Environ Contam Toxicol 105(5):798–805. https://doi.org/10.1007/s00128-020-02983-x
Zhao L, Feng Y, Xu ZJ, Zhang NY, Zhang WP, Zuo G, Khalil MM, Sun LH (2021) Selenium mitigated aflatoxin B1-induced cardiotoxicity with potential regulation of 4 selenoproteins and ferroptosis signaling in chicks. Food Chem Toxicol 154:112320. https://doi.org/10.1016/j.fct.2021.112320
Li S, Sun WJ, Zhang K, Zhu JW, Jia XT, Guo XQ, Zhao QY, Tang CH, Yin JD, Zhang JM (2021) Selenium deficiency induces spleen pathological changes in pigs by decreasing selenoprotein expression, evoking oxidative stress, and activating inflammation and apoptosis. J Anim Sci Biotechnol 12(1):65. https://doi.org/10.1186/s40104-021-00587-x
Kelly E, Greene CM, Carroll TP, McElvaney NG, O’Neill SJ (2009) Selenoprotein S/SEPS1 modifies endoplasmic reticulum stress in Z variant alpha1-antitrypsin deficiency. J Biol Chem 284(25):16891–16897. https://doi.org/10.1074/jbc.M109.006288
Du SQ, Liu HM (1800) Huang KX (2010) Influence of SelS gene silence on beta-Mercaptoethanol-mediated endoplasmic reticulum stress and cell apoptosis in HepG2 cells. Biochim Biophys Acta 5:511–517. https://doi.org/10.1016/j.bbagen.2010.01.005
Speckmann B, Gerloff K, Simms L, Oancea I, Shi W, McGuckin MA, Radford-Smith G, Khanna KK (2014) Selenoprotein S is a marker but not a regulator of endoplasmic reticulum stress in intestinal epithelial cells. Free Radic Biol Med 67:265–277. https://doi.org/10.1016/j.freeradbiomed.2013.11.001
Shchedrina VA, Everley RA, Zhang Y, Gygi SP, Hatfield DL, Gladyshev VN (2011) Selenoprotein K Binds Multiprotein Complexes and Is Involved in the Regulation of Endoplasmic Reticulum Homeostasis. J Biol Chem 286(50):42937–42948. https://doi.org/10.1074/jbc.M111.310920
Wang LY, Yin JF, Yang B, Qu CJ, Lei J, Han J, Guo X (2020) Serious Selenium Deficiency in the Serum of Patients with Kashin-Beck Disease and the Effect of Nano-Selenium on Their Chondrocytes. Biol Trace Elem Res 194(1):96–104. https://doi.org/10.1007/s12011-019-01759-7
Singh P, Marcu KB, Goldring MB, Otero M (2019) Phenotypic instability of chondrocytes in osteoarthritis: on a path to hypertrophy. Ann N Y Acad Sci 1442(1):17–34. https://doi.org/10.1111/nyas.13930
Gao Y, Walder K, Sunderland T, Kantham L, Feng HC, Quick M, Bishara N (2003) Andrea de Silva, Augert G, Tenne-Brown J, Collier GR (2003) Elevation in Tanis Expression Alters Glucose Metabolism and Insulin Sensitivity in H4IIE Cells. Diabetes 52(4):929–934. https://doi.org/10.2337/diabetes.52.4.929
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81872565).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, H., Li, Z., Liu, Y. et al. Effects of Selenoprotein S Knockdown on Endoplasmic Reticulum Stress in ATDC5 Cells and Gene Expression Profiles in Hypertrophic Chondrocytes. Biol Trace Elem Res 201, 1965–1976 (2023). https://doi.org/10.1007/s12011-022-03313-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03313-4