Abstract
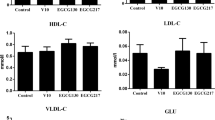
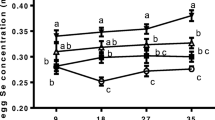
This study evaluated the effect of epigallocatechin-3-gallate (EGCG) alleviating the reduction of antioxidant capacity induced by dietary vanadium (V) in the liver, kidney, and ovary of laying hens. Furthermore, Kelch-like ECH-associated protein 1(Keap1)-nuclear factor erythroid 2-related factor 2 (Nrf2)-small Maf proteins (sMaf) pathway was explored to reveal the molecular mechanism. A total of 768 40-week-old Hyline-Brown laying hens were randomly allocated to 4 groups with 8 pens per group and 24 hens per pen. The experimental groups were as follows: control (basal diet); V15, control + 15 mg/kg V; EGCG150, control + 150 mg/kg EGCG; V15 + EGCG150, V15 + 150 mg/kg EGCG. Our results revealed that dietary EGCG supplementation completely alleviated the V-induced reductions of hen-day egg production, average egg weight, Haugh unit, albumen height, eggshell strength, and eggshell thickness. Dietary EGCG supplementation completely prevented the V-induced reductions of serum follicle-stimulating hormone and luteinizing hormone levels. Besides, dietary EGCG supplementation reversed the V-induced increments of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine (Cr), and uric acid (UA). In addition, dietary EGCG supplementation partially alleviated the V-induced reductions of the enzyme activities and gene expressions of superoxidative dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GSH-Px). Furthermore, dietary EGCG supplementation partially alleviated the V-induced reductions of Nrf2 and sMaf gene expressions, and the increments of Keap1 gene expression. In summary, EGCG partially alleviated V-induced reduction of antioxidant capacity through Keap1-Nrf2-sMaf pathway in the liver, kidney, and ovary of laying hens.




Similar content being viewed by others
References
Domingo JL (2000) Vanadium and diabetes. What about vanadium toxicity? Mol Cell Biochem 203(1-2):185–187. https://doi.org/10.1023/a:1007067011338
Mukherjee B, Patra B, Mahapatra S, Banerjee P, Tiwari A, Chatterjee M (2004) Vanadium-an element of atypical biological significance. Toxicol Lett 150(2):135–143. https://doi.org/10.1016/j.toxlet.2004.01.009
Ma Y, Zhu MK, Miao LP, Zhang XY, Dong XY, Zou XT (2018) Mercuric chloride induced ovarian oxidative stress by suppressing Nrf2-Keap1 signal pathway and its downstream genes in laying hens. Biol Trace Elem Res 185(1):186–196. https://doi.org/10.1007/s12011-018-1244-y
Scibior A, Zaporowska H, Ostrowski J, Banach A (2006) Combined effect of vanadium (V) and chromium (III) on lipid peroxidation in liver and kidney of rats. Chem Biol Interact 159(3):213–222. https://doi.org/10.1016/j.cbi.2005.11.008
Kurt O, Ozden TY, Ozsoy N, Tunail S, Can R, Akev N, Yanardag R (2011) Influence of vanadium supplementation on oxidative stress factors in the muscle of STZ-diabetic rats. BioMetals 24(5):943–949. https://doi.org/10.1007/s10534-011-9452-3
Storey KB (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29(12):1715–1733. https://doi.org/10.1006/bmme.1996.0088
Wu G, Fang Y, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134(3):489–492. https://doi.org/10.1093/jn/134.3.489
Wang J, Yuan Z, Zhang K, Ding X, Bai S, Zeng Q, Peng H, Celi P (2018) Epigallocatechin-3-gallate protected vanadium-induced eggshell depigmentation via P38MAPK-Nrf2/HO-1 signaling pathway in laying hens. Poult Sci 97(9):3109–3118. https://doi.org/10.3382/ps/pey165
Tiedge M, Lortz S, Drinkgern J, Lenzen S (1997) Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46(11):1733–1742. https://doi.org/10.2337/diabetes.46.11.1733
Kwak MK, Itoh K, Yamamoto M, Sutter T, Kensler TW (2001) Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dithiole-3-thione. Mol Med 7(2):135–145. https://doi.org/10.1177/1532673X12437555
Zhang DD (2006) Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38(4):769–789. https://doi.org/10.1080/03602530600971974
Sriram N, Kalayarasan S, Sudhandiran G (2009) Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm Pharmacol Ther 22(3):22–236. https://doi.org/10.1016/j.pupt.2008.12.010
Caetano M, Padrino L, Gutiérrez H, Fernández AJ, Castillo J (1998) Trace level vanadium determination using thermal lens spectrophotometry. Mikrochim Acta 128:169–175. https://doi.org/10.1007/BF01243045
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Domingo JL, Gomez M, Llobet JM, Corbella J, Keen CL (1991) Oral vanadium administration to streptozotocin-diabetic rats has marked negative side-effects which are independent of the form of vanadium used. Toxicology 66(3):279–287. https://doi.org/10.1016/0300-483X(91)90199-B
Crans DC, Smee JJ, Gaidamauskas E, Yang LQ (2004) The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev 104(2):849–902. https://doi.org/10.1002/chin.200420288
Davis EG, Miles RD, Butcher GD, Comer CW (2002) Effects of dietary vanadium on performance and immune response of commercial egg-type laying hens. J Appl Anim Res 22(1):113–124. https://doi.org/10.1080/09712119.2002.9706386
Yuan ZH, Zhang KY, Ding XM, Luo YH, Bai SP, Zeng QF, Wang JP (2016) Effect of tea polyphenols on production performance, egg quality, and hepatic antioxidant status of laying hens in vanadium-containing diets. Poult Sci 95(7):1709–1717. https://doi.org/10.3382/ps/pew097
Stevenson MJ, Uyeda KS, Harder NHO, Heffern MC (2019) Metal-dependent hormone function: the emerging interdisciplinary field of metalloendocrinology. Metallomics 11(1):85–110. https://doi.org/10.1039/C8MT00221E
Graham JD, Clarke CL (1997) Physiological action of progesterone in target tissues. Endocr Rev 18(4):502–519. https://doi.org/10.1210/edrv.18.4.0308
Petersen SL, Ottem EN, Carpenter CD (2003) Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod 69(6):1771–1778. https://doi.org/10.1095/biolreprod.103.019745
Scibior A, Zaporowska H (2007) Effects of vanadium(V) and/or chromium(III) on L-ascorbic acid and glutathione as well as iron, zinc, and copper levels in rat liver and kidney. J Toxicol Environ Health Part A 70(8):696–704. https://doi.org/10.1080/15287390601187906
Tipoe GL, Leung TM, Liong EC, Lau TYH, Fung ML, Nanji AA (2010) Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 273(1-3):45–52. https://doi.org/10.1016/j.tox.2010.04.014
Mielgo-Ayuso J, Barrenechea L, Alcorta P, Larrart E, Margareto J, Labayen I (2014) Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br J Nutr 111(7):1263–1271. https://doi.org/10.1017/S0007114513003784
Kakuta Y, Okumi M, Isaka Y, Tsutahara K, Abe T, Yazawa K, Ichimaru N, Matsumura K, Hyon SH, Takahara S, Nonomura N (2011) Epigallocatechin-3-gallate protects kidneys from ischemia reperfusion injury by HO-1 upregulation and inhibition of macrophage infiltration. Transpl Int 24(5):514–522. https://doi.org/10.1111/j.1432-2277.2011.01224.x
Imura H, Shimada A, Naota M, Morita T, Togawa M, Hasegawa T, Seko Y (2013) Vanadium toxicity in mice. Toxicol Pathol 41(6):842–856. https://doi.org/10.1177/0192623312467101
Na HK, Surh TJ (2008) Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol 46(4):1271–1278. https://doi.org/10.1016/j.fct.2007.10.006
Cui X, Gong J, Han H, He L, Teng Y, Tetley T, Sinharay R, Chung KF, Islam T, Gillilang F, Grady S, Garshick E, Li Z, Zhang JJ (2018) Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J Thorac Dis 10(5):3088–3097. https://doi.org/10.21037/jtd.2018.05.92
Finkel T, Holbrook NJ (2018) Oxidants, oxidative stress and the biology of aging. Nature 408(6809):239–247. https://doi.org/10.1038/35041687
Tuzcu M, Sahin N, Karatepe M, Cikim G, Kilinc U, Sahin K (2008) Epigallocatechin-3-gallate supplementation can improve antioxidant status in stressed quail. Br Poult Sci 49(5):643–648. https://doi.org/10.1080/00071660802298336
Kanna PS, Mahendrakumar CB, Chatterjee M, Hemalatha P, Datta S, Chakraborty P (2003) Vanadium inhibits placental glutathione S-transferase (GST-P) positive foci in 1, 2-dimethyl hydrazine induced rat colon carcinogenesis. J Biochem Mol Toxicol 17(6):357–365. https://doi.org/10.1002/jbt.10099
Sahin K, Orhan C, Tuzcu M, Ali S, Sahin N, Hayirli A (2010) Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult Sci 89(10):2251–2258. https://doi.org/10.3382/ps.2010-00749
Motohashi H, Yamamoto M (2004) Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10(11):549–557. https://doi.org/10.1016/j.molmed.2004.09.003
Tkachev VO, Menshchikova EB, Zenkov NK (2011) Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry 76(4):407–422. https://doi.org/10.1134/S0006297911040031
Wang JP, He KR, Ding XM, Luo YH, Bai SP, Zeng QF, Su ZW, Xuan Y, Zhang KY (2016) Effect of dietary vanadium and vitamin C on egg quality and antioxidant status in laying hens. J Anim Physiol Anim Nutr 100(3):440–447. https://doi.org/10.1111/jpn.12377
Funding
This study was supported by the Earmarked Fund for PhD Research Start-up Fund of Henan University of Science and Technology (No. 13480086).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All process in the present study was approved by the Animal Ethics and Welfare Committee of Henan University of Science and Technology (Luoyang, China). The experiment was carried out according to the Guiding Principles in the Use of Animals in Toxicology, adopted by the Chinese Society of Toxicology.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 324 kb)
Rights and permissions
About this article
Cite this article
Ma, Y., Shi, Y., Wu, Q. et al. Epigallocatechin-3-gallate Alleviates Vanadium-Induced Reduction of Antioxidant Capacity via Keap1-Nrf2-sMaf Pathway in the Liver, Kidney, and Ovary of Laying Hens. Biol Trace Elem Res 199, 2707–2716 (2021). https://doi.org/10.1007/s12011-020-02398-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02398-z