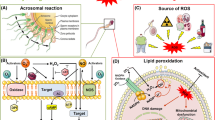
Abstract
Present study was undertaken on buck spermatozoa to investigate the effect of mercuric chloride on functional dynamics of buck spermatozoa. Four different concentrations (0.031, 0.125, 0.25 and 1.25 μg/mL) of mercuric chloride, which were 1/40th, 1/10th, 1/5th and equivalent to the LC50 value of HgCl2, were selected for studying their effect following in vitro exposure for 15 min and 3 h. Exposure of spermatozoa to 0.031 μg/mL mercuric chloride for 3 h resulted in significant (p < 0.05) decrease in sperm motility, sperm having intact membrane, intact acrosome and high mitochondrial trans-membrane potential. However, following exposure to higher concentrations (0.25, 1.25 μg/mL), similar results were observed even after 15 min of exposure. HgCl2 significantly (p < 0.05) increased the levels of malondialdehyde and reactive oxygen species and significantly (p < 0.05) decreased total antioxidant capacity and superoxide dismutase activity in spermatozoa within 15 min of exposure. Mercuric chloride-treated spermatozoa did not show capacitation, rather exhibited spontaneous acrosome reaction along with significant increase in intracellular Ca2+ and cAMP levels. Immuno-blotting of semen samples of control and 0.031 μg/mL mercury-treated groups showed low intensity bands of p55, p70, p80, p105 and p190 kDa tyrosine phosphorylation proteins while higher concentration-treated groups showed no such bands. Our findings evidently suggest that mercuric chloride even at 0.031 μg/mL adversely affected sperm functions, inhibited tyrosine phosphorylation proteins and capacitation due to oxidative stress. Spontaneous acrosome reaction (AR) in mercury-treated spermatozoa may possibly be due to increase in intracellular Ca2+ and cAMP levels, and capacitation failure may be due to inhibition of tyrosine phosphorylation of proteins.





Similar content being viewed by others
References
Abou-haila, Tulsiani DR (2009) Signal transduction pathways that regulate sperm capacitation and the acrosome reaction. Arch Biochem Biophys 485:72–81
Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79:829–843
Agarwal A, Virk G, Du C, Ong Plessis SS (2014) Effect of oxidative stress on male reproduction. World J Men's Health 32:1–17
Agency for Toxic Substances and Disease Registry (ATSDR) (2009) Toxicological profile for mercury. Public Health Service, U.S. Department of Health and Human Services, Atlanta
Aitken RJ (2017) Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev 84:1039–1052
Aitken RJ, Buckingham DW, West KM (1992) Reactive oxygen species and human spermatozoa: analysis of the cellular mechanisms involved in luminol- and lucigenin-dependent chemiluminescence. J Cell Physiol 151:466–477
Amidi F, Pazhohan A, Nashtaei MS, Khodarahmian M, Nekoonam S (2016) The role of antioxidants in sperm freezing: a review. Cell Tissue Bank 17:745–756
Arabi M (2006) The role of mercury in the etiology of sperm dysfunction in Holstein bulls. Asian-Aust J Anim Sci 19:335–340
Arabi M, Heydarnejad MS (2007) In-vitro mercury exposure on spermatozoa from normospermic individuals. Pak J Biol Sci 10:2448–2453
Arito H. and Takahashi M (1991) Effect of methylmercury on sleep patterns in the rat. In Advances in mercury toxicology (pp. 381–394). Springer, Boston, MA
Ashrafi H, Kohram H, Tayefi-Nasrabadi (2013) Antioxidant effects of bovine serum albumin on kinetics, microscopic and oxidative characters of cryopreserved bull spermatozoa. Span J Agric Res 11:695–701
Au DW, Chiang MW, Wu RS (2000) Effects of cadmium and phenol on motility and ultrastructure of sea urchin and mussel spermatozoa. Arch Environ Contam Toxicol 38:455–463
Baldi E, Han CJ, Lawrie HL, Mack SR, Zaneveld LJ (1991) Modulation of the human sperm acrosome reaction by effectors of the adenylate cyclase/cyclic AMP second messenger pathway. Exp Zool 258:113–125
Baldi E, Luconi M, Bonaccorsi L, Muratori M, Forti G (2000) Intracellular events and signaling pathways involved in sperm acquisition of fertilizing capacity and acrosome reaction. Front Bio Sci 1:110–123
Belyaeva AE, Glazunov V, Korotkov S (2004) Cd2+-promoted mitochondrial permeability transition: a comparison with other heavy metals. Acta Biochim Pol 51:545–551
Belyaeva AE, Dorota DM, Wieckowski R, Wojtczak L (2008) Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol Appl Pharm 231:34–42
Berlin M, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662
Berlin M, Zalups RK, Fowler BA (2007) “Mercury” in handbook on the toxicology of metals. G. F. Nordberg, B. A
Boujbiha MA, Hamden K, Guermazi F, Bouslama A, Omezzine A (2009) Testicular toxicity in mercuric chloride treated rats: association with oxidative stress. Reprod Toxicol 28:81–89
Branco V, Caito S, Farina M, da Rocha T, Aschner J, Carvalho MC (2017) Biomarkers of mercury toxicity: past, present, and future trends. J Toxicol Environ Health 20:119–154. https://doi.org/10.1080/10937404.2017.1289834
Buffone MG, Wertheimer EV, Visconti PE, Krapf D (2014) Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim Biophys Acta 1842:2610–2620
Chauhan DS, Swain DK, Yadav HP, Shah N, Nakade UP, Singh VK, Nigam R, Yadav S, Garg SK (2016) Functional and molecular characterization of voltage gated sodium channel Nav 1.8 in bull spermatozoa. Theriogenology 90:210–218
Corvelo TC, Oliveira EA, de Parijos AM, de Oliveira CS, de Loiola RSP (2014) Monitoring mercury exposure in reproductive aged women inhabiting the tapajos river basin, Amazon. Bull Environ Contam Toxicol 1:42–46
Creasy DM (2001) Pathogenesis of male reproductive toxicity. Toxicol Pathol 29:64–76
Cummins JM, Fleming AD, Crozet N, Kuehl TJ, Kosower NS, Yanagimachi R (1986) Labelling of living mammalian spermatozoa with the fluorescent thiol alkylating agent, mono bromobimane (MB): immobilization upon exposure to ultraviolet light and analysis of acrosomal status. J Exp Zool 237:375–382
El-Desoky GE, Bashandy SA, Alhazza IM, Al-Othman ZA, Aboul-Soud MA, Yusuf K (2013) Improvement of mercuric chloride-induced testis injuries and sperm quality deteriorations by Spirulina platensis in rats. PLoS One 28(8):e59177
Emokpae Nigeria MA, Adobor C, Ibadin K (2016) Seminal plasma levels of lead and mercury in infertile males in Benin City. Int J Med Res Health Sci 5:1–6
Ernst E, Lauritsen JG (1991) Effect of organic and inorganic mercury on human sperm motility. Pharmacol Toxicol 68:440–444
Esteves SC, Sharma RK, Thomas AJ, Agarwal A (1996) Suitability of the hypo-osmotic swelling test for assessing the viability of cryopreserved sperm. Fertil Steril 66:798–804
Esteves SC, Sharma RK, Thomas AJ, Agarwal A (1998) Cryopreservation of human spermatozoa with pentoxifylline improves the post-thaw agonist-induced acrosome reaction rate. Hum Reprod 13:3384–3389
Gupta RC, Milatovic D, Lall R, Srivastava (2018) Mercury. Vet Toxicol:455–462. https://doi.org/10.1016/b978-0-12-811410-0.00031-3
Hancock JL (1952) The morphology of bull spermatozoa. J Exp Biol 29:445–453
He Y, Zou Q, Chen H, Weng S, Luo T, Zeng X (2016) Lead inhibits human sperm functions by reducing the levels of intracellular calcium, cAMP, and tyrosine phosphorylation. Tohoku J Exp Med 238:295–303. https://doi.org/10.1620/tjem.238.295
Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C (2003) DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod Biomed 7:477–484
Henson MC, Piasek M, Chedrese PJ, Castracane VD (2016) Metal Toxicity in Mammalian Reproduction. Endocrine Toxicology 19:256
Jakub S, Anna S, Agata NS, Katarzyna K, Andrzej K (2017) Occupational mercury vapours poisoning with a respiratory failure, pneumome diastinum and severe quadriparesis. SAGE open medical case reports-5 2050313X17695472
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Mohd Q, Haq R (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16:29592–29630
Jeyendran RS, Vander V, Perez-Pelaez HH, Crabo MBG, Zanevld LJD (1984) Development of an assay to access the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 70:219–228
Kajari D, Luna S, Gagan C (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 37:201–204
Kazantzis GF, Nordberg ED (2007) Diagnosis and treatment of metal poisoning-general aspects. In: Handbook on The Toxicology of Metals, chapter 15, 3rd edn. Elsevier, NY, pp 313–314
Kim SH, Sharma RP (2004) Mercury-induced apoptosis and necrosis in murine macrophages: role of calcium-induced reactive oxygen species and p38 mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol 196:47–57
Krasznai Z, Morisawa MS, Krasznai ZT, Tron L, Gaspar R, Marian T (2003) Role of ion channels and membrane potential in the initiation of carp sperm motility. Aquat Living Resour 16:445–449
Lamirande De E, Gagnon C (2002) The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol Hum Reprod 8:124–135
Leoni G, Bogliolo L, Deiana G, Berlinguer F, Rosati I, Pintus PP, Ledda S, Naitana S (2002) Influence of cadmium exposure on in vitro ovine gamete dysfunction. ReprodToxicol 16:371–377
Li LZ, Yan F, Miao L, Jia NT, Ju FZ, Bin W, Yi HG, Huijuan S (2017) Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS One 12(11):e018672
Liguori L, De Lamirande E, Minelli A, Gagnon C (2004) Various protein kinases regulate human sperm acrosome reaction and the associated phosphorylation of Tyr residues and of the Thr-Glu-Tyr motif. Mol Hum Reprod 11:211–221
Lirui W, Li Y, Fu J, Linqing N, Zhen Z, Li Q, Yang S, Li X (2016) Cadmium inhibits mouse sperm motility through inducing tyrosine phosphorylation in a specific subset of proteins. Reprod Toxicol 63:96–106
Mansour RT, Serour MG, Abbas AM (2008) The impact of spermatozoa preincubation time and spontaneous acrosome reaction in intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril 90:584–591
Mendiola J, Moreno JM, Roca M (2011) Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: a pilot study. Environ Health 10:6. https://doi.org/10.1186/1476-069X-10-6
Mendoza C, Carreras A, Tesarik MJ (1992) Distinction between the true acrosome reactions by a one step staining method using Pisum sativum agglutinin. J Reprod Fertil 95:755–763
Middendorff R, Davidoff MS, Behrends S, Mewe M, Miethens A, Muller D (2000) Multiple roles of the messenger molecule cGMP in testicular function. Andrologia 32:55–59
Mortimer D, Testart J, Frydman R (1985) From the semen to oocyte: the long route in vivo and in vitro short cut in human in-vitro fertilization: actual problems and prospects. Elsevier Science Publishers, Amsterdam, p 93
Mostafa KM, Thomas MB, Karle NM (1987) Effects of methyl mercury on testicular functions in Macaca fascicularis monkeys. Pharmacol Toxicol 60:29–36. https://doi.org/10.1111/j.1600-0773.1987.tb01715
Oliveira H, Spano M, Santos C, Lourdes MP (2009) Lead chloride affects sperm motility and acrosome reaction in mice. Cell Biol Toxicol 25:341–353
Rana SV (2014) Perspectives in endocrine toxicity of heavy metals—a review. Biological trace element research 1;160(1):1–4
Rao M, Gangadharan B (2008) Antioxidative potential of melatonin against mercury induced intoxication in spermatozoa in vitro. Toxicol in Vitro 22:935–942
Rao MV, Sharma PN (2001) Protective effect of vitamin-E against mercuric chloride reproductive toxicity in male mice. Reprod Toxicol 15(6):705–712
Rathi R, Colenbrander B, Bevers MM, Gadella BM (2001) Evaluation of in vitro capacitation of stallion spermatozoa. Biol Reprod 65:462e70
Rice MK, Ernest MW, Miaozong JW, Chris G, Eric RB (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47:74–83. https://doi.org/10.3961/jpmph.47.2.74
Rossato M, Virgilio FD, Rizzuto R, Galeazzi C, Foresta C (2001) Intracellular calcium store depletion and acrosome reaction in human spermatozoa: role of calcium and plasma membrane potential. Mol Hum Reprod 7(2):119–128
Roychoudhury S, Massanyi P, Bulla J, Choudhury MD, Lukac N, Filipejova T, Almasiova V (2010) Cadmium toxicity at low concentration on rabbit spermatozoa motility, morphology and membrane integrity in vitro. J Environ Sci Heal, Part A 45(11):1374–1383. https://doi.org/10.1080/10934529.2010.500909
Selvaraju S, Somashekar L, Krishnan BB, Parthipan S, Pushparani GA, Arangasamy D, Rajendran JP (2016) Relationship between seminal plasma tuberoinfundibular peptide of 39 residues and sperm functional attributes in buffalo (Bubalus bubalis). Reprod Fertil Dev 28(10):1622–1630. https://doi.org/10.1071/RD15008
Shafiq-ur-Rehman SR, Chandra O, Abdulla M (1995) Evaluation of malondialdehyde as an index of lead damage in rat brain homogenates. Bio Metals 8:275–279
Shenker BJ, Tai LG, Irving MS (2008) Mercury-induced apoptosis in human lymphoid cells: evidence that the apoptotic pathway is mercurial species dependent. Environ Res Sect-A 4:89–99
Swain DK, Yadav S, Singh SK (2017) Effect of four different in vitro incubation temperatures on functional dynamics, process of capacitation and apoptosis in goat spermatozoa. Small Rumin Res 147:120–124
Urner F, Sakkas D (2003) Protein phosphorylation in mammalian spermatozoa. Reproduction 125:17–26
Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS (1995) Capacitation of mouse spermatozoa. II Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. J Develop 121(4):1139–1150
Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB (2002) Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol 53:133–150
Yanagimachi R (2011) Mammalian sperm acrosome reaction: where does it begin before fertilization? Biol Reprod 85:4–5. https://doi.org/10.1095/biolreprod.111.092601
Zheng R, Canyang LI (2009) Effect of lead on survival, locomotion and sperm morphology of Asian earthworm, Pheretimaguillelmi. Int J Environ Sci 21:691–695
Acknowledgements
We are also thankful to Professor and Head, Department of Pharmacology and Toxicology, for providing the necessary facilities in ICAR Niche Area of Excellence Laboratories. Laboratory facilities provided by the former and present Heads, Department of Veterinary Physiology, and technical assistance provided by Dr. Brijesh Yadav and Dr. Mukul Anand are also thankfully acknowledged.
Funding
Financial assistance was provided by Dean, College of Biotechnology, to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Committee Approval
The guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals Govt. of India were followed as per the approval of Institutional Animal Ethics Committee (Approval No. 110/IAEC/16, dated: 16-09-2016).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 494 kb)
Rights and permissions
About this article
Cite this article
Kushawaha, B., Yadav, R.S., Swain, D.K. et al. Mercury-Induced Inhibition of Tyrosine Phosphorylation of Sperm Proteins and Altered Functional Dynamics of Buck Spermatozoa: an In Vitro Study. Biol Trace Elem Res 198, 478–492 (2020). https://doi.org/10.1007/s12011-020-02077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02077-z