Abstract
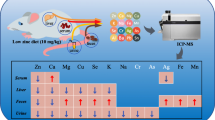
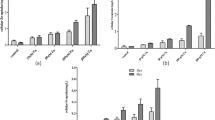
Zinc plays an essential role in various fundamental biological processes. The focus of this research was to investigate the dosage effect of zinc glycine chelate (Zn-Gly) on zinc metabolism and the gene expression of zinc transporters in intestinal segments. A total of 30 4-week-old SD rats were randomized into five treatment groups. The basal diets for each group were supplemented with gradient levels of Zn (0, 30, 60, 90, and 180 mg/kg) from Zn-Gly. After 1-week experiment, the results showed that serum and hepatic zinc concentration were elevated linearly with supplemental Zn levels from 0 to 180 mg Zn/kg. Serum Cu-Zn SOD activities resulted in a significant (P < 0.01) quadratic response and reached the peak when fed 60 mg Zn/kg. There were linear responses to the addition of Zn-Gly from 0 to 180 mg Zn/kg on Cu-Zn SOD and AKP activities in the liver. In the duodenum, MT1 mRNA was upregulated with the increasing dietary Zn-Gly levels and reached the peak of 180 mg Zn/kg (P < 0.05). Zip4 mRNA expression was downregulated with the increasing zinc levels (P < 0.05) in both duodenum and jejunum. In the jejunum, Zip5 mRNA expression in 60 mg Zn/kg was higher compared with other groups (P < 0.05). ZnT1 mRNA in duodenum was numerically increased with the rising levels of zinc content and was significantly higher (P < 0.05) with 180 mg Zn/kg. In the duodenum, adding 60 or 90 mg Zn/kg increased PepT1 expression, but in the jejunum, 60 mg Zn/kg did not differ from 0 added Zn. In summary, there is a dose-dependent effect of dietary Zn-Gly on serum and hepatic zinc levels and the activities of Cu-Zn SOD and AKP on rats. Dietary Zn-Gly has a certain effect on MT1, Zip4, Zip5, and ZnT1 expression, which expressed differently in intestinal segments with different levels of Zn-Gly load. Besides, Zn-Gly also could regulate PepT1 expression in intestinal segments.





Similar content being viewed by others
References
Dolegowska B, Machoy Z, Chlubek D (2003) Changes in the content of zinc and fluoride during growth of the femur in chicken. Biol Trace Elem Res 91(1):67–76
Wang LF, Yang GY, Yang GQ (2014) Effect of zinc source on the expression of ZIPII transporter genes in Guanzhong dairy goats. Animal Feed Sci. Tech. 195:129–135
Zhao CY, Tan SX, Xiao XY (2014) Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol Trace Elem Res 160:361–367
Huang YL, Lu L, Li SF, et al. (2009) Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed a conventional corn-soybean meal diet. J. Ani. Sci. 87(6):2038–2046
Wang JH, Wu CC, Feng J (2011) Effect of dietary antibacterial peptide and zinc-methionine on performance and serum biochemical parameters in piglets. Czeth J Anim Sci 56(1):30–36
Cao J, Henry PR, Guo R, et al. (2000) Chemical characteristics and relative bioavailability of supplemental organic zinc sources for poultry and ruminants. J Anim Sci 78:2039–2054
Hudson BP, Dozier WA, Wilson JL (2005) Broiler live performance response to dietary zinc source and the influence of zinc supplementation in broiler breeder diets. Anim. Feed Sci. Tech. 118(3–4):329–335
Soni N, Mishra SK, Swain R, et al. (2013) Bioavailability and immunity response in broiler breeders on organically complexed zinc supplementation. Food Nutr Sci 4:1293–1300
Wang Y, Tang JW, Ma WQ, et al. (2010) Dietary zinc glycine chelate on growth performance, tissue mineral concentration, and serum enzyme activity in weanling piglets. Biol Trace Elem Res 133(3):325–334
Feng J, Ma WQ, Niu HH, et al. (2010) Effect of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol Trace Elem Res 133(2):203–211
Ma WQ, Niu HH, Feng J (2011) Effect of zinc glycine chelate on oxidative stress, contents of trace elements, and intestinal morphology in broilers. Biol Trace Elem Res 142(3):546–556
O’Dell, 1984. Bioavailability of trace elements. Nutr Rev, 42(9): 301–308.
Kambe T, Hashimoto A, Fujimoto S (2014) Current understanding of zip and ZnT zinc transporters in human health and disease. Cell Mol Life Sci 71(17):3281–3295
Yue M, Fang SL, Zhuo Z, et al. (2014) Zinc glycine chelate absorption characteristics in Sprague Dawley rat. J Anim Physiol Anim Nutr (Berl). doi:10.1111/jpn.12255
Hill GM, Miller ER (1983) Effect of dietary zinc levels on the growth and development of the gilt. J.Anim.Sci. 57:106
Gonzalez, F., Esther Ferez-Vidal, M., Arias, J.M., et al., 1994. Partial purification and biochemical properties of acid and alkaline phosphatases from Myxococcus coralloides. D. Journal of Applied Bacteriology. J Appl Microbiol, 77(5): 567–573.
Wang SH, Chen JC (2005) The protective effect of chitin and chitosan against Vibrio alginolyticus in white shrimp, Litopenaeus vannamei. Fish & Shellfish Immunology 19(3):191–204
Martin L, Pieper R, Kroger S (2012) Influence of age and Enterococcus faecium NCIMB 10415 on development of small intestinal digestive physiology in piglets. Anim. Feed Sci Tech. 175(1–2):65–75
Yu ZP, Le GW, Shi YH (2005) Effect of zinc sulphate and zinc methionine on growth, plasma growth hormone concentration, growth hormone receptor and insulin-like growth factor-1 gene expression in mice. Clin Exp Pharmacol Physiol 32(4):273–278
Case CL, Carlson MS (2002) Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J.Anim.Sci. 80(7):1917–1924
Martin L, Pieper R, Schunter N, et al. (2013) Performance, organ zinc concentration, jejunal brush border membrane enzyme activities and mRNA expression in piglets fed with different levels of dietary zinc. Arch Anim Nutr 67(3):248–261
Midilli M, Salman M, Muglali OH, et al. (2014) The effect of organic or inorganic zinc and microbial phytase, alone or in combination, on the performance, biochemical parameters and nutrient utilization of broilers fed a diet low in available phosphorus. International Journal of Biological, Veterinary, Agricutural and Food Engineering 20(1):99–106
Sun JY, Jing MY, Weng XY, et al. (2005) Effects of dietary zinc levels on the activities of enzymes, weights of organs, and the concentration of zinc and copper in growing rats. Biol Trace Elem Res 107(2):153–165
Zhang CS, Zhu WJ, Guan XM, et al. (2006) Effects of interaction between dietary zinc and vitamin A in broilers on performance, immunity, ALP and CuZn-SOD activity and serum insulin concentration. World J Zool 1(1):17–23
Revy PS, Jondreville C, Dourmad JY, et al. (2006) Assessment of dietary zinc requirement of weaned piglets fed diets with or without microbial phytase. J Anim Physiol Anim Nutr 90(1–2):50–59
Miles AT, Hawksworth GM, Beattie JH, et al. (2000) Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol 35(1):35–70
Pfaffl MW, Windisch W (2003) Influence of zinc deficiency on the mRNA expression of zinc transporters in adult rats. J Trace Elem Med Biol 17(2):97–106
McMahon RJ, Cousins RJ (1998) Regulation of zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci U S A 95:4841–4846
Masaki H, Ochial Y, Okano Y, et al. (2007) A zinc(II)-glycine complex is an effective inducer of metallothionein and removes oxidative stress. J. Der. Sci. 45(1):73–75
Tohru Y, Hiroki O, Miho N, et al. (2012) In vitro study on the transport of zinc across intestinal epithelial cells using caco-2 monolayers and isolated rat intestinal membranes. Biol. Pharm. Bull. 35(4):588–593
Romeo A, Vacchina V, Legros S, et al. (2014) Zinc fate in animal husbandry systems. Royal Society of Chemistry 6(11):1999–2009
Dufner-Beattie J, Kuo YM, Gitschier J, et al (2004) The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem 279(47):49082–49090
Weaver BP, Dufner BJ, Kambe T, et al. (2007) Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse SLC 39a4 and SLC 39a5 zinc transporters (Zip4 and Zip5). Biol.Chem. 388(12):1301–1312
Jeong J, Eide DJ (2013) The SLC39 family of zinc transporters. Mol. Aspects of Medi. 34(2–3):612–619
Wang XX, Zhou B (2010) Dietary zinc absorption: a play of Zip and ZnTs in the gut. IUBMB Life 62(3):176–182
Yu YY, Kirschke CP, Huang L (2007) Immunohistochemical analysis of ZnT1, 4, 5, 6, and 7 in the mouse gastrointestinal tract. J Histochem Cytochem 55:223–234
Liuzzi JP, Blanchard RK, Cousins RJ (2001) Differential regulation of zinc transporter 1, 2, and 4 mRNA expression by dietary zinc in rats. J Nutr 131:46–52
Ashmead H (1991) Comparative intestinal absorption and subsequent metabolism of metal amino acid chelates and inorganic metal salts. Biol Tr Elem Res 306-319
Pizarro F, Olivares M, Hertrampf E, et al. (2002) Iron bis-glycine chelate competes for the nonheme-iron absorption pathway. Amer J Clin Nutr 76:577–581
Liao ZC, Guan WT, Chen F, et al. (2014) Ferrous bisglycinate increased iron transportation through DMT1 and PepT1 in pig intestinal cells compared with ferrous sulphate. J Anim Feed Sci 23:153–159
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31472102), a Key Science Project “973” Award from National Science and Technology Committee (Grant No. 2012CB124705).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, D., Hu, Q., Fang, S. et al. Dosage Effect of Zinc Glycine Chelate on Zinc Metabolism and Gene Expression of Zinc Transporter in Intestinal Segments on Rat. Biol Trace Elem Res 171, 363–370 (2016). https://doi.org/10.1007/s12011-015-0535-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0535-9