Abstract
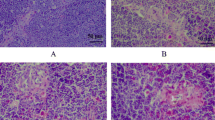
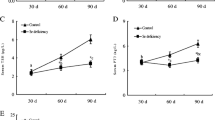
Selenium is an essential trace element possessing immune-stimulatory properties. The purpose of this 42-day study was to investigate the effects of excess dietary sodium selenite on immune function by determining morphological changes and apoptosis of bursa of Fabricius. Three hundred 1-day-old Avian broilers were fed on a basic diet (0.2 ppm selenium) or the same diet amended to contain 1, 5, 10, and 15 ppm selenium supplied as sodium selenite (n = 60/group). Relative weight of bursa was significantly decreased in the 1, 5, 10, and 15 ppm groups at 28 days of age, when compared with that of 0.2 ppm group. Pathological lesions were progressed with the dietary Se level increased. The gross lesions of bursa involved obvious atrophy with decreased volume and pale color. Histopathologically, decreased number of lymphocytes and loosely packed lymphocytes appeared in the medulla and cortex in the follicles. Ultrastructurally, mitochondria injury and increased apoptotic cells with condensed nuclei were observed. In comparison to that of control group, excess Se (5, 10, and 15 ppm) intake increased the percentage of Annexin V positive cells, as measured by flow cytometry. Terminal deoxynucleotidyl transferase 2′-deoxyuridine 5′-triphosphate nick end-labeling assay showed that there were increased frequencies of apoptotic cells in 10 and 15 ppm selenium groups. These data suggest that Se supplementation with sodium selenite should be carefully evaluated as excess selenium (more than 5 ppm) intake could cause profound immunologic inhibition.





Similar content being viewed by others
References
R.C McKenzie, G.J Becket, and J.R Arthur. Effect of selenium on immunity and aging, in Selenium Its Molecular Biology and Role in Human Health. D.L Hatfield, M.J Berry, V.N Gladyshev, ed., Springer, New York, pp. 311–322 (2006).
J.N Thompson, and M.L Scott. Impaired lipid and vitamin E absorption related to atrophy of the pancreas in selenium-deficient chicks. J. Nutr. 100:797–809(1970).
M.F Raisbeck, E.R Dahl, D.A Sanchez, E.L Belden and D O’Toole. Naturally occurring selenosis in Wyoming. J Vet Diagn Invest. 5, 84–87 (1993).
D O’Toole and M.F Raisbeck. Pathology of experimentally induced chronic selenosis (alkali disease) in yearling cattle. J Vet Diagn Invest. 7, 364–373 (1995).
K.E Panter, W.J Hartley, L.F James, H.F Mayland, B.L Stegelmeier and P.O Kechele. Comparative toxicity of selenium from Seleno-DL-methionine, Sodium Selenate, and Astragalus bisulcatus in pigs. Toxicol. Sci. 32, 217–223 (1996).
D.E Green and P.H Albers. Diagnostic criteria for selenium toxicosis in aquatic birds: histologic lesions. J. Wildl. Dis. 33(3), 385–404 (1997).
J.R Arthur, R.C McKenzie, G.J Beckett. Selenium in the immune system. J Nutr. 133, S1457–1459 (2003).
J.E Spallholz, J.L Martin, M.L Gerlach and R.H Heinzerling. Enhanced immunoglobulin M and immunoglobulin G antibody titers in mice fed selenium. Infect Immun. 8(5), 841–842 (1973).
S.M Hegazy and Y Adachi. Comparison of the effects of dietary selenium, zinc, and selenium and zinc supplementation on growth and immune response between chick groups that were inoculated with Salmonella and aflatoxin or Salmonella. Poult Sci. 79, 331–335 (2000).
L Vega, M Rodríguez-Sosa, E.A García-Montalvo, L.M Del Razo and E Elizondo. Non-optimal levels of dietary selenomethionine alter splenocyte response and modify oxidative stress markers in female mice. Food and Chemical Toxicology. 45(7), 1147–1153 (2007).
C Riddell. Avian histopathology. In: Lymphoid system. C Riddell, ed., Lawrence, KS: American Association of Avian Pathologists, pp. 7–17 (1987)
R.J Turner, L.E Wheatley, N.F.G Beck. Stimulatory effects of selenium on mitogen responses in lambs. Veterinary Immunology and Immunopathology. 8, 119–124 (1985).
R.R Watson, S Moriguchi, B McRae, L Tobin, J.C Mayberry and D Lucas. Effects of selenium in vitro on human T-lymphocyte functions and K-562 tumor cell growth. J Leukoc Biol. 39, 447–456 (1986).
J.A Marsh, G.F Combs, M.E Whitacre and R.R Dietert. Effect of selenium and vitamin E dietary deficiencies on chick lymphoid organ development. Proceedings of the Society for Experimental Biology and Medicine. 182, 425–436 (1986).
H.M Cui. The histopathologic study of selenium deficiency in chicks. Acta Veterinaria et Zootechnica Sinica. 1: 52–56 (1988).
T.S Kim, B.Y Yun and I.Y Kim. Induction of the mitochondrial permeability transition by selenium compounds mediated by oxidation of the protein thiol groups and generation of the superoxide. Biochemical Pharmacology. 66, 2301–2311(2003).
T.S Kim, D.W Jeong, B.Y Yun and I.C Kim. Dysfunction of rat liver mitochondria by selenite: induction of mitochondrial permeability transition through thiol-oxidation. Biochemical and Biophysical Research Communication. 294, 1130–1137 (2002).
M Weiller, M Latta, M Kresse, R Lucas and A Wendel. Toxicity of nutritionally available selenium compounds in primary and transformed hepatocytes. Toxicol. 201, 21–30 (2004).
C.L Shen, W Song and B.C Pence. Interactions of selenium compounds with other antioxidants in DNA damage and apoptosis in human normal keratinocytes. Cancer Epidemiol Biomarkers Prev. 10, 385–390 (2001).
V Cryns and J Yuan. Proteases to die for. Genes & Development. 12, 1551–1570 (1998).
D.R Green. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 102:1–4 (2000).
S.V.S Rana. Metals and apoptosis: recent development. Journal of Trace Elements in Medicine and Biology. 22, 262–284. doi:10.1016/j.jtemb. 2008.08.002.
Acknowledgments
This work was supported by the Education Department and Scientific Department of Sichuan Province (2006A007) and Program for Changing Scholars and Innovative Research Team in University (PCSIRT 0835).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, X., Cui, Y., Cui, W. et al. The Decrease of Relative Weight, Lesions, and Apoptosis of Bursa of Fabricius Induced by Excess Dietary Selenium in Chickens. Biol Trace Elem Res 131, 33–42 (2009). https://doi.org/10.1007/s12011-009-8345-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8345-6