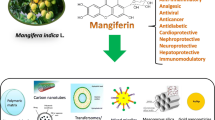
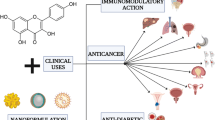
Abstract
In order to cure a range of ailments, scientists have investigated a number of bioactive antioxidant compounds produced from natural sources. Mangiferin, a C-glycosyl xanthone-structured yellow polyphenol, is abundant in mangoes and other dietary sources. In-depth examinations found that it is effective in the treatment of a variety of disorders due to its antiviral, anti-inflammatory, antiproliferative, antigenotoxic, antiatherogenic, radioprotective, nephroprotective, antihyperlipidemic, and antidiabetic properties. However, it is recognised that mangiferin’s poor bioavailability, volatility, and limited solubility restrict its therapeutic usefulness. Over time, effective solutions to these problems have arisen in the shape of effective delivery methods. The current articles present a summary of the several researches that have updated Mangiferin’s biopharmaceutical characteristics. Additionally, strategies for enhancing the bioavailability, stability, and solubility of this phytodrug have been discussed. This review provides detailed information on the development of innovative Mangiferin delivery methods such as nanoparticles, liposomes, micelles, niosomes, microspheres, metal nanoparticles, and complexation, as well as its therapeutic applications in a variety of sectors. This article provides effective guidance for researchers who desire to work on the formulation and development of an effective delivery method for improved magniferin therapeutic effectiveness.






Similar content being viewed by others
Data Availability
Not applicable.
References
Admapriya, K., Dutta, A., Chaudhuri, S., & Dutta, D. (2012). Microwave assisted extraction of mangiferin from Curcuma amada. Biotech, 2(1), 27–30.
Jyotshna; Srivastava, P., Killadi, B., & Shanker, K. (2015). Uni-dimensional double development HPTLC-densitometry method for simultaneous analysis of mangiferin and lupeol content in mango (Mangifera indica) pulp and peel during storage. Food chem, 176, 91–98.
Wu, J. F., Chen, S. B., Gao, J. C., Song, H. L., Wu, L. J., Chen, S. L., & Tu, P. F. (2008). Xanthone glycosides from herbs of Polygala Hongkongensis Hemsl and their antioxidant activities. Journal of Asian Natural Products Research, 10(7–8), 673–678.
Rashid, K., & Sil, P. C. (2017). Identification and extraction of antidiabetic antioxidants from natural sources. Elsevier Inc. 1, 3–111.
Walia, V., Chaudhary, S. K., & Kumar Sethiya, N. (2021). Therapeutic potential of mangiferin in the treatment of various neuropsychiatric and neurodegenerative disorders. Neurochemistry International, 143, 104939.
Shan, T., Ma, Q., Guo, K., Liu, J., Li, W., Wang, F., & Wu, E. (2011). Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Current Molecular Medicine, 11(8), 666–677.
Lei, J., Zhou, C., Hu, H., Hu, L., Zhao, M., Yang, Y., Chuai, Y., Ni, J., & Cai, J. (2012). Mangiferin aglycone attenuates radiation-induced damage on human intestinal epithelial cells. Journal of Cellular Biochemistry, 113(8), 2633–2642.
Dou, W., Zhang, J., Ren, G., Ding, L., Sun, A., Deng, C., Wu, X., Wei, X., Mani, S., & Wang, Z. (2014). Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-κB and MAPK signaling inactivation. International Immunopharmacology, 23(1), 170–178.
du Plessis-Stoman, D., du Preez, J., & van de Venter, M. (2011). Combination treatment with oxaliplatin and mangiferin causes increased apoptosis and downregulation of NFκB in cancer cell lines. AJTCAM, 8(2), 177–184.
Satish Rao, B. S., Sreedevi, M. V., & Nageshwar Rao, B. (2009). Cytoprotective and antigenotoxic potential of mangiferin, a glucosylxanthone against cadmium chloride induced toxicity in HepG2 cells. Food and Chemical Toxicology, 47(3), 592–600.
Jang, M. H., Piao, X. L., Kim, J. M., Kwon, S. W., & Park, J. H. (2008). Inhibition of cholinesterase and amyloid-beta aggregation by resveratrol oligomers from Vitis amurensis. Phyther Res, 22(4), 544–549.
Jangra, A., Arora, M. K., Kisku, A., & Sharma, S. (2021). The multifaceted role of mangiferin in health and diseases: A review. Adv Tradit Med, 21, 619–643.
Ma, H., Chen, H., Sun, L., Tong, L., & Zhang, T. (2014). Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia, 93, 54–61.
Bhattacharyya, S., Ahmmed, S. M., Saha, B. P., Mukherjee, P. K., & Mukherjee (2014). Soya phospholipid complex of mangiferin enhances its hepatoprotectivity by improving its bioavailability and pharmacokinetics. Journal of the Science of Food and Agriculture, 94, 1380–1388.
Mei, S., Perumal, M., Battino, M., Kitts, D. D., Xiao, J., Ma, H., & Chen, X. (2021). Mangiferin: A review of dietary sources, absorption, metabolism, bioavailability, and safety. Critical Reviews in Food Science and Nutrition, 63(18), 3046–3064.
Andreu, G. P., Delgado, R., Velho, J. A., Curti, C., & Vercesi, A. E. (2005). Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe 2+-citrate. European Journal of Pharmacology, 513, 47–55.
Quadri, F., Telang, M., & Mandhare, A. (2019). Therapeutic and cosmetic applications of mangiferin: An updated patent review (patents published after 2013). Expert Opinion on Therapeutic Patents, 19, 463–479.
Bulugonda, R. K., Kumar, K. A., Gangappa, D., Beeda, H., Philip, G. H., Muralidhara Rao, D., & Faisal, S. M. (2017). Mangiferin from Pueraria tuberosa reduces inflammation via inactivation of NLRP3 inflammasome. Scientific Reports, 7, 1–14.
Picot, M. C. N., Zengin, G., Mollica, A., Stefanucci, A., Carradori, S., & Mahomoodally, M. F. (2017). In vitro and in silico studies of mangiferin from Aphloia theiformis on key enzymes linked to diabetes type 2 and associated complications. Medicinal Chemistry, 13, 1–2.
Sekar, M. (2015). Molecules of interest – Mangiferin – A review. Annu Res Rev Biol, 5, 307–320.
Phillips, B. A., & Danner, F. J. (1989). Xanthones in Methods in plant biochemistry, 1st Edition, Elsevier, 1, 175–178
Matkowski, A., Piotr, K., Edyta, G., & Dorota, W. (2013). Mangiferin – A bioactive xanthonoid, not only from mango and not just antioxidant. Mini-Reviews Med Chem, 13, 439–455.
Vasilev, N., Nedialkov, P., Ionkova, I., & Ninov, S. (2004). HPTLC densitomeric determination of justicidin B in Linum in vitro cultures. Die Pharmazie, 59, 528–529.
Dimitrov, M., Nikolova, I., Benbasat, N., Kitanov, G., & Danchev, N. (2011). Acute toxicity, antidepressive and MAO inhibitory activity of mangiferin isolated from Hypericum aucheri. Biotechnology and Biotechnological Equipment, 25, 2668–2671.
Tangah, J., Bajau, F. E., Jilimin, W., Chan, H. T., Wong, S. K., & Chan, E. W. C. (2017). Phytochemistry and pharmacology of Mangifera pajang: An iconic fruit of Sabah, Malaysia. Syst Rev Pharm, 8, 86–91.
Chauhan, R. S., & Dutt, P. (2013). Swertia ciliata - A new source of mangiferin, amaroswerin and amarogentin. J Biol Act Prod from Nat, 3, 161–165.
Sethiya, N. K., Trivedi, A., & Mishra, S. H. (2015). Rapid validated high performance thin layer chromatography method for simultaneous estimation of mangiferin and scopoletin in Canscora Decussata (South Indian Shankhpushpi) extract. Rev Bras Farmacogn, 25, 193–198.
Bera, S., Chaudhuri, S., & Dutta, D. (2015). Assessment of free-radical scavenging activities of mangiferin from Curcuma amada obtained by non-conventional extraction methods: A comparative study. Indian J Biotechnol, 14, 179–185.
Rammohan, A., Gunasekar, D., Reddy, N. V., Vijaya, T., Deville, A., & Bodo, B. (2015). Structure elucidation and antioxidant activity of the phenolic compounds from rhynchosia suaveolens. Natural Products Communications, 10, 609–611.
Bhatia, V. K., Ramanathan, J. D., & Seshadri, T. R. (1967). Constitution of mangiferin. Tetrahedron, 23, 1363–1368.
Aritomi, M., & Kawasaki, T. (1970). Position isomer of mangiferin, from Anemarrhena asphodeloides BUNGE. Chemical & Pharmaceutical Bulletin, 18, 2327–2333.
Aritomi, M., Kawasaki, T. (1970). A mangiferin monomethyl ether from Mangifera indica L. Chemical & Pharmaceutical Bulletin, 18, 2224–2234.
Ehianeta, T. S., Laval, S., & Yu, B. (2016). Bio- and chemical syntheses of mangiferin and congeners. Biofactors, 42, 445–458.
Fujita, M., & Inoue, T. (1980). Biosynthesis of mangiferin in Anemarrhena asphodeloides BUNGE. I. The origin of the xanthone nucleus. Chemical & Pharmaceutical Bulletin, 28, 2476–2481.
Fujita, M., & Inoue, T. (1981). Further studies on the biosynthesis of mangiferin in Anemarrhena asphodeloides: Hydroxylation of the shikimate-derived ring. Phytochemistry, 20, 2183–2185.
Kitumrungsart, R. P. P., & Suntornwat, O. (2011). Mangniferin and antioxidant capacity from mango leaves extract. Thai J Agric Sci, 44, 62–66.
Fujita, M., & Inoue, T. (1982). Studies on the constituents of Iris florentina L II C-glycisides of xanthones and flavones from the leaves. Chemical & Pharmaceutical Bulletin, 30, 2342–2348.
Richardson, P. M. (1984). The taxonomic significance of xanthones in ferns. Biochemical Systematics and Ecology, 12, 1–6.
Vyas, A., Syeda, K., Ahmad, A., Padhye, S., & Sarkar, F. H. (2012). Perspectives on medicinal properties of mangiferin. Mini-Reviews Med Chem, 12, 412–425.
Khurana, R. K., Kaur, R., Lohan, S., Singh, K. K., & Singh, B. (2016). Mangiferin: A promising anticancer bioactive. Pharm Pat Anal, 5, 169–181.
Rocha Ferreira, F., Barros Valentim, I., Catarí Ramones, E., Salles Trevisan, M., Olea Azar, C., Pérez Cruz, F., & de Caxico, F. (2013). y Fonseca Goulart, M. Antioxidant activity of the mangiferin inclusion complex with β-cyclodextrin. Food Sci Technol, 51, 129–134.
Hou, S., Wang, F., Li, Y., Wang, M., Sun, D., & Sun, C. (2012). Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chemistry, 132, 289–294.
Núñez Selles, A. J., Daglia, M., & Rastrelli, L. (2016). The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. Biofactors, 42, 475–491.
Khurana, R. K., Gaspar, B. L., Welsby, G., Katare, O. P., Singh, K. K., & Singh, B. (2018). Improving the biopharmaceutical attributes of mangiferin using vitamin E-TPGS co-loaded self-assembled phosholipidic nano-mixed micellar systems. Drug Deliv Transl Res, 8, 617–632.
Sah, A. K., Vyas, A., Suresh, P. K., & Gidwani, B. (2018). Application of nanocarrier-based drug delivery system in treatment of oral cancer, Artif. Cells. Nanomedicine Biotechnol, 46, 650–657.
Afsharzadeh, M., Hashemi, M., Mokhtarzadeh, A., Abnous, K., & Ramezani, M. (2018). Recent advances in co-delivery systems based on polymeric nanoparticle for cancer treatment, Artif. Cells. Nanomedicine Biotechnol, 46, 1095–1110.
Badwaik, H. R., Nakhate, K., Kumari, L., & Sakure, K. (2018). Oral delivery of proteins and polypeptides through polysaccharide nanocarriers in Polysaccharide-Based Nano-Biocarrier Drug Deliv, 1–24.
Badwaik, H. R., Al Hoque, A., Kumari, L., Sakure, K., Baghel, M., & Giri, T. K. (2020). Moringa gum and its modified form as a potential green polymer used in biomedical field. Carbohydrate Polymers, 249, 32933701.
Venditti, I. (2019). Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J King Saud Univ - Sci, 31, 398–411.
Kumari, L., Badwaik, H. R. (2019). Polysaccharide-based nanogels for drug and gene delivery in: Polysaccharide carrier for drug delivery. Elsevier, 497–558.
Kumari, L., Baghel, M., Panda, S., Sakure, K., Giri, T. K., Badwaik, H., & Chemistry (2021). Biological activities, and uses of moringa gum. Gums, resins and latexes of Plant Origin: Chemistry. Springer nature. Biological Activities and Uses.
George, A., Shah, P. A., & Shrivastav, P. S. (2019). Natural biodegradable polymers-based nano-formulations for drug delivery: A review. International Journal of Pharmaceutics, 561, 244–264.
Baghel, M., Sakure, K., Giri, T. K., Maiti, S., Nakhate, K. T., Ojha, S., Sharma, C., Agrawal, Y., Goyal, S., & Badwaik, H. (2023). Carboxymethylated gums and derivatization: Strategies and significance in drug delivery and tissue engineering. Pharmaceuticals, 16, 776.
Akbari, A., S., Shaddel, R., & Jafari, S. M. (2020). Encapsulation of food bioactives and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocoll, 105, 105774–105791.
Badwaik, H. R., Kumari, L., Maiti, S., Sakure, K., Ajazuddin, Nakhate, K. T., Tiwari, V., & Giri, T. K. (2022). A review on challenges and issues with carboxymethylation of natural gums: The widely used excipients for conventional and novel dosage forms. International Journal of Biological Macromolecules, 209, 2197–2212.
Wang, M., Zhang, Z., Huo, Q., Wang, M., Sun, Y., Liu, H., Chang, J., He, B., & Liang, Y. (2022). Targeted polymeric nanoparticles based on mangiferin for enhanced protection of pancreatic β-cells and type 1 diabetes mellitus efficacy. Acs Applied Materials & Interfaces, 14, 11092–11103.
Razura-Carmona, F. F., Pérez-Larios, A., González-Silva, N., Herrera-Martínez, M., Medina-Torres, L., Sáyago-Ayerdi, S. G., & Sánchez-Burgo, J. A. (2019). Mangiferin-loaded polymeric nanoparticles: Optical characterization, effect of antitopoisomerase I, and cytotoxicity. Cancers (Basel), 11(12), 1–17.
Samadarsi, R., & Dutta, D. (2019). Design and characterization of mangiferin nanoparticles for oral delivery. Journal of Food Engineering, 247, 80–94.
Singh, A. K., Chaurasiya, A., Awasthi, A., Mishra, G., Asati, D., Khar, R. K., & Mukherjee, R. (2009). Oral bioavailability enhancement of exemestane from self-micro emulsifying drug delivery system (SMEDDS). An Official Journal of the American Association of Pharmaceutical Scientists, 10(3), 906–916.
Bartoszewski, R., Hering, A., Marszałł, M., Stefanowicz Hajduk, J., Bartoszewska, S., Kapoor, N., Kochan, K., & Ochocka, R. (2014). Mangiferin has an additive effect on the apoptotic properties of hesperidin in Cyclopia sp. tea extracts. PLoS One. 9(3), e-92128.
Bezerra, Francisco, W. A., Fechine, Lillian, M. U. D., Lopes, Karen, P. S., de Sousa, A. F.; do, Nascimento, G. O., Amaral, H. H., de Leal, S., Trevisan, L. K. A. M., Ribeiro, M. T. S., & Ricardo, M. E. N. P. (2019). Nágila M.P.S. α-Glucosidase inhibitory activity of mangiferin-loaded F127/PEG micellar system, Mater. Lett. 255, 126522.
Bilia, A. R., Bergonzi, M. C., Guccione, C., Manconi, M., Fadda, A. M., & Sinico, C. (2016). Vesicles and micelles: Two versatile vectors for the delivery of natural products. Journal of Drug Delivery Science and Technology, 32, 241–255.
Biswas, S., Kumari, P., Lakhani, P. M., & Ghosh, B. (2016). Recent advances in polymeric micelles for anti-cancer drug delivery. European Journal of Pharmaceutical Science, 83, 184–202.
Feng, R., Song, Z., & Zhai, G. (2012). Preparation and in vivo pharmacokinetics of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles. Int J Nanomedicine, 7, 4089–4098.
Zhou, S., Deng, X., & Yang, H. (2003). Biodegradable poly(ε-caprolactone)-poly(ethylene glycol) block copolymers: Characterization and their use as drug carriers for a controlled delivery system. Biomaterials, 24, 3563–3570.
Dutra, L. M. U., Cavalcante, I. M., De Brito, D. H. A., Vieira, I. G. P., Trevisan, M. T. S., Ribeiro, M. E. N. P., Yeates, S. G., & Ricardo, N. M. P. S. (2017). Synergistic effect in drug solubility by new binary micelles of poly(ϵ-caprolactone)-poly(ethylene oxide) and F127®. Journal of the Brazilian Chemical Society, 28, 1341–1346.
Moura, J. U., Barbosa, G. M., Genro, C., Hernández, R. D., Izquierdo, S. S., Gomes, P., Fagan, S. B., & Raffin, R. P. (2014). Mangiferin-loaded polymeric nanocapsules. J Nanopharmaceutics Drug Deliv, 2, 87–92.
Sguizzato, M., Ferrara, F., Hallan, S. S., Baldisserotto, A., Drechsler, M., Malatesta, M., Costanzo, M., Cortesi, R., Puglia, C., Valacchi, G., & Esposito, E. (2021). Ethosomes and transethosomes for mangiferin transdermal delivery. Antioxidants, 10(5), 768–779.
Abdulbaqi, I. M., Darwis, Y., Khan, N. A. K., Assi, R. A., & Khan, A. A. (2016). Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. International Journal of Nanomedicine, 11, 2279–2304.
Song, H., Wen, J., Li, H., Meng, Y., Zhang, Y., Zhang, N., & Zheng, W. (2019). Enhanced transdermal permeability and drug deposition of rheumatoid arthritis via sinomenine hydrochloride-loaded antioxidant surface transethosome. International Journal of Nanomedicine, 14, 3177–3188.
Ascenso, A., Raposo, S., Batista, C., Cardoso, P., Mendes, T., Praça, F. G., Bentley, M. V. L. B., & Simões, S. (2015). Development, characterization, and skin delivery studies of related ultradeformable vesicles: Transfersomes, ethosomes, and transethosomes. International Journal of Nanomedicine, 10, 5837–5851.
Ghasemiyeh, P., & Mohammadi-Samani, S. (2018). Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res Pharm Sci, 13, 288–303.
Allaw, M., Pleguezuelos-Villa, M., Manca, M. L., Caddeo, C., Aroffu, M., Nacher, A., Diez-Sales, O., Saurí, A. R., Ferrer, E. E., Fadda, A. M., & Manconi, M. (2020). Innovative strategies to treat skin wounds with mangiferin: Fabrication of transfersomes modified with glycols and mucin. Nanomedicine: The Official Journal of the American Academy of Nanomedicine, 15, 1671–1685.
Benson, H. A. (2006). Transfersomes for transdermal drug delivery. Expert Opinion on drug Delivery, 3(6), 727–737.
Ahad, A., Al-Saleh, A. A., Al-Mohizea, A. M., Al-Jenoobi, F. I., Raish, M., Yassin, A. E. B., & Alam, M. A. (2018). Formulation and characterization of Phospholipon 90 G and tween 80 based transfersomes for transdermal delivery of eprosartan mesylate. Pharmaceutical Development and Technology, 23, 787–793.
Larrucea, E., Arellano, A., Santoyo, S., & Ygartua, P. (2001). Combined effect of oleic acid and propylene glycol on the percutaneous penetration of tenoxicam and its retention in the skin. European Journal of Pharmaceutics and Biopharmaceutics, 52, 113–119.
Pedersen, L. K., & Jemec, G. B. E. (1999). Plasticising effect of water and glycerin on human skin in vivo. Journal of Dermatological Science, 19, 48–52.
Bansil, R., & Turner, B. S. (2006). Mucin structure, aggregation, physiological functions and biomedical applications. Current Opinion in Colloid & Interface Science, 11, 164–170.
Souto, E. B., Doktorovova, S., Gonzalez-Mira, E., Egea, M. A., & Garcia, M. L. (2010). Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Current Eye Research, 35, 537–552.
Souto, E. B., & Müller, R. H. (2010). Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. Handbook of Experimental Pharmacology, 197, 115–141.
Liu, R., Liu, Z., Zhang, C., & Zhang, B. (2012). Nanostructured lipid carriers as novel ophthalmic delivery system for mangiferin: Improving in vivo ocular bioavailability. Journal of Pharmaceutical Sciences, 101, 2271–2280.
Santonocito, D., Vivero-Lopez, M., Lauro, M. R., Torrisi, C., Castelli, F., Sarpietro, M. G., & Puglia, C. (2022). Design of nanotechnological carriers for ocular delivery of mangiferin: Preformulation study, Molecules 27(4), 1328–1342.
Aburahma, M. H., & Badr-Eldin, S. M. (2014). Compritol 888 ATO: A multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals, Expert Opinion on Drug Delivery. Expert Opinion on Drug Delivery, 11, 1865–1883.
ICCVAM, Test Method Evaluation Report. (2010). Current validation status of in vitro test methods proposed for identifying eye injury hazard potential of chemicals and products. (Vol. 2). Interagency Coordinating Committee on the Validation of Alternative Methods Nationa.
Zhou, Q., Hou, K., & Fu, Z. T. M. (2022). Mangiferin-loaded SLNs: Preparation, characterization, and application in A549 lung cancer cell. Drug Design, Development and Therapy, 16, 1767–1778.
Mao, X., Liu, L., Cheng, L., Cheng, R., Zhang, L., Deng, L., Sun, X., Zhang, Y., Sarmento, B., & Cui, W. (2019). Adhesive nanoparticles with inflammation regulation for promoting skin flap regeneration. Journal of Controlled Release : Official Journal of the Controlled Release Society, 297, 91–101.
Angelova-Fischer, I., Rippke, F., Richter, D., Filbry, A., Arrowitz, C., Weber, T., Fischer, T. W., & Zillikens, D. (2018). Stand-alone emollient treatment reduces flares after discontinuation of topical steroid treatment in atopic dermatitis: A double-blind, randomized, vehicle-controlled, left-right comparison study. Acta Dermato Venereologica, 98, 517–523.
Ring, J., Alomar, A., Bieber, T., Deleuran, M., Fink-Wagner, A., Gelmetti, C., Gieler, U., Lipozencic, J., Luger, T., Oranje, A. P., Schäfer, T., Schwennese, T., Seidenari, S., Simon, D., Ständer, S., Stingl, G., Szalai, S., Szepietowski, J. C., Taïeb, A., & Werfel, T. (2012). Global Allergy and Asthma European Network (GA2LEN), Guidelines for treatment of atopic eczema (atopic dermatitis) part I. JEADV, 26(8), 1045–1060.
Eichenfield, L. F., Tom, W. L., Berger, T. G., Krol, A., Paller, A. S., Schwarzenberger, K., Bergman, J. N., Chamlin, S. L., Cohen, D. E., Cooper, K. D., Cordoro, K. M., Davis, D. M., Feldman, S. R., Hanifin, J. M., Margolis, D. J., Silverman, R. A., Simpson, E. L., Williams, H. C., Elmets, C. A., Block, J., & Sidbury, R. (2014). Guidelines of care for the management of atopic dermatitis: Sect. 2. Management and treatment of atopic dermatitis with topical therapies. Journal of the American Academy of Dermatology, 71(1), 116–132.
Pleguezuelos-Villa, M., Diez-Sales, O., Manca, M. L., Manconi, M., Sauri, A. R., Escribano-Ferrer, E., & Nácher, A. (2020). Mangiferin glycethosomes as a new potential adjuvant for the treatment of psoriasis. International Journal of Pharmaceutics, 573, 118844.
Singh, Y., Meher, J. G., Raval, K., Khan, F. A., Chaurasia, M., Jain, N. K., & Chourasia, M. K. (2017). Nanoemulsion: Concepts, development and applications in drug delivery. Journal of Controlled Release : Official Journal of the Controlled Release Society, 252, 28–49.
Khichariya, A., Jeswani, G., Choudhary, R., Alexander, A., Nakhate, K. T., & Ramchandra Badwaik, H. (2022). Formulation of plumbagin-loaded microemulsion: Evaluation of anti-rheumatoid efficacy in Wistar rat model. Journal of Molecular Liquids, 363, 1–7.
Montes de Oca-Ávalos, J. M., Candal, R. J., & Herrera, M. L. (2017). Nanoemulsions: Stability and physical properties. Curr Opin Food Sci, 16, 1–6.
Pleguezuelos-Villa, M., Nácher, A., Hernández, M. J., Ofelia Vila Buso, M. A., Ruiz Sauri, A., & Díez-Sales, O. (2019). Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. International Journal of Pharmaceutics, 564, 299–307.
Sharma, H., Mishra, P. K., Talegaonkar, S., & Vaidya, B. (2015). Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov Today, 20, 1143–1151.
Parveen, S., Sur, T., Sarkar, S., & Roy, R. (2023). Antagonist impact of selenium-based nanoparticles against Mycobacterium tuberculosis. Applied Biochemistry and Biotechnology, 195(6), 3606–3614.
Fan, M., Han, Y., Gao, S., Yan, H., Cao, L., Li, Z., Liang, X. J., & Zhang, J. (2020). Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics, 10, 494–4957.
Connor, E. E., Mwamuka, J., Gole, A., Murphy, C. J., & Wyatt, M. D. (2005). Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small (Weinheim An Der Bergstrasse, Germany), 1, 325–327.
Muddineti, O. S., Ghosh, B., & Biswas, S. (2015). Current trends in using polymer coated gold nanoparticles for cancer therapy. International Journal of Pharmaceutics, 484, 252–267.
Ahmad, N., Mohd, S., Rizvi, D., Sahai, N., & Dutta, R. (2016). Biosynthesis, characterization of gold nanoparticles using M. indica leaf extract and their anticancer activity. International Journal of Nanomedicine, 2, 7–11.
Muralikrishna, T., Malothu, R., Pattanayak, M., & Nayak, P. L. (2014). Green synthesis of gold nanoparticles using Mangifera indica (mango leaves) aqueous extract. World J Nano Sci Technol, 3, 66–73.
Philip, D. (2010). Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim Acta - Part A Mol Biomol Spectrosc, 77, 807–810.
Al-Yasiri, A. Y., Khoobchandani, M., Cutler, C. S., Watkinson, L., Carmack, T., Smith, C. J., Kuchuk, M., Loyalka, S. K., Lugão, A. B., & Katti, K. V. (2017). Mangiferin functionalized radioactive gold nanoparticles (MGF-198AuNPs) in prostate tumor therapy: Green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalt Trans, 46, 14561–14571.
Khoobchandani, M., Khan, A., Katti, K. K., Thipe, V. C., Al-Yasiri, A. Y., MohanDoss, D. K. D., Nicholl, M. B., Lugão, A. B., Hans, C. P., & Katti, K. V. (2021). Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy. Scientific Reports, 11, 1–30.
Aboyewa, J. A., Sibuyi, N. R. S., Meyer, M., & Oguntibeju, O. O. (2021). Gold nanoparticles synthesized using extracts of cyclopia intermedia, commonly known as honeybush, amplify the cytotoxic effects of doxorubicin. Nanomaterials, 11, 1–16.
Ji, S. R., Liu, C., Zhang, B., Yang, F., Xu, J., Long, J., Jin, C., Fu, D. L., Ni, Q. X., & Yu, X. J. (2010). Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta - Rev Cancer, 1806, 29–35.
Bhattacharya, K., Mukherjee, S. P., Gallud, A., Burkert, S. C., Bistarelli, S., Bellucci, S., Bottini, M., Star, A., & Fadeel, B. (2016). Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomedicine Nanotechnology Biol Med, 12, 333–351.
Son, K. H., Hong, J. H., & Lee, J. W. (2016). Carbon nanotubes as cancer therapeutic carriers and mediators. Int J Nanomedicine, 11, 5163–5185.
Harsha, P. J., Thotakura, N., Kumar, M., Sharma, S., Mittal, A., Khurana, R. K., Singh, B., Negi, P., & Raza, K. (2019). A novel PEGylated carbon nanotube conjugated mangiferin: An explorative nanomedicine for brain cancer cells. Journal of Drug Delivery Science and Technology. 53, 101186.
Raza, K., Kumar, N., Misra, C., Kaushik, L., Guru, S. K., Kumar, P., Malik, R., Bhushan, S., & Katare, O. P. (2016). Dextran-PLGA-loaded docetaxel micelles with enhanced cytotoxicity and better pharmacokinetic profile. International Journal of Biological Macromolecules, 88, 206–212.
Hoyos-Arbeláez, J., García, G., Arévalo, F. J., Vázquez, M. V., Fernández, H., & Granados, S. G. (2018). Electrochemical determination of mangiferin using glassy carbon electrodes modified with carbonaceous nanomaterials. Journal of Electroanalytical Chemistry, 808, 1–7.
Montes, A., Wehner, L., Pereyra, C., & De La Ossa (2016). E.J.M. Mangiferin nanoparticles precipitation by supercritical antisolvent process. Journal of Supercritical Fluids, 112, 44–50.
Telange, D. R., Sohail, N. K., Hemke, A. T., Kharkar, P. S., & Pethe, A. M. (2021). Phospholipid complex-loaded self-assembled phytosomal soft nanoparticles: Evidence of enhanced solubility, dissolution rate, ex vivo permeability, oral bioavailability, and antioxidant potential of mangiferin. Drug Deliv Transl Res, 11, 1056–1083.
Khurana, R. K., Bansal, A. K., Beg, S., Burrow, A. J., Katare, O. P., Singh, K. K., & Singh, B. (2017). Enhancing biopharmaceutical attributes of phospholipid complex-loaded nanostructured lipidic carriers of mangiferin: Systematic development, characterization and evaluation. International Journal of Pharmaceutics, 518, 289–306.
Verma, P., Kuwahara, Y., Mori, K., Raja, R., & Yamashita, H. (2020). Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale, 12, 1–5.
Zhao, D., Huo, Q., Feng, J., Chmelka, B. F., & Stucky, G. D. (1998). Nonionic triblock and star diblock copolymer and oligomeric sufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. Journal of the American Chemical Society, 120, 6024–6036.
Pontes Silva, C. R., da Rocha Ferreira, F., Dresch Webler, G., Da Silva, A. O. S., De Abreu, F. C., & Fonseca, E. J. S. (2017). Encapsulation of mangiferin in ordered mesoporous silica type SBA-15: Synthesis and characterization. Mater Res Express, 6(4), 5402.
Sarfraz, M., Khan, A., Batiha, G. E., Akhtar, M. F., Saleem, A., Ajiboye, B. O., Kamal, M., Ali, A., Alotaibi, N. M., Aaghaz, S., Siddique, M. I., & Imran, M. (2023). Nanotechnology-based drug delivery approaches of mangiferin: Promises, reality and challenges in cancer chemotherapy. Cancers (Basel), 15(16), 4194.
Sarkar, A., Sreenivasan, Y., Ramesh, G. T., & Manna, S. K. (2004). β-D-glucoside suppresses tumor necrosis factor-induced activation of nuclear transcription factor κB but potentiates apoptosis. Journal of Biological Chemistry, 279, 33768–33781.
Sahoo, B. K., Zaidi, A. H., Gupta, P., Mokhamatam, R. B., Raviprakash, N., Mahali, S. K., & Manna, S. K. (2015). A natural xanthone increases catalase activity but decreases NF-kappa B and lipid peroxidation in U-937 and HepG2 cell lines. European Journal of Pharmacology, 764, 520–528.
Yoshimi, N., Matsunaga, K., Katayama, M., Yamada, Y., Kuno, T., Qiao, Z., Hara, A., Yamahara, J., & Mori, H. (2001). The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Letters, 163, 163–170.
Dilshara, M. G., Kang, C. H., Choi, Y. H., & Kim, G. Y. (2015). Mangiferin inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 expression and cellular invasion by suppressing nuclear factor-κB activity. Bmb Reports, 48, 559–564.
Rajendran, P., Ekambaram, G., & Sakthisekaran, D. (2008). Cytoprotective effect of mangiferin on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Basic & Clinical Pharmacology & Toxicology, 103, 137–142.
Takeda, T., Tsubaki, M., Sakamoto, K., Ichimura, E., Enomoto, A., Suzuki, Y., Itoh, T., Imano, M., Tanabe, G., Muraoka, O., Matsuda, H., Satou, T., & Nishida, S. (2016). Mangiferin, a novel nuclear factor kappa B-inducing kinase inhibitor, suppresses metastasis and tumor growth in a mouse metastatic melanoma model. Toxicol Appl Pharmacol, 306, 105–112.
Li, H., Huang, J., Yang, B., Xiang, T., Yin, X., Peng, W., Cheng, W., Wan, J., Luo, F., Li, H., & Ren, G. (2013). Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicol Appl Pharmacol, 272, 180–190.
Jung, J. S., Jung, K., Kim, D. H., & Kim, H. S. (2012). Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: Involvement of PI3K/Akt and MAPK signaling pathways. Pharmacological Research, 66, 95–103.
Li, M., Ma, H., Yang, L., & Li, P. (2016). Mangiferin inhibition of proliferation and induction of apoptosis in human prostate cancer cells is correlated with downregulation of B-cell lymphoma-2 and upregulation of microRNA-182. Oncol Lett, 11, 817–822.
Katayama, K., Noguchi, K., & Sugimoto, Y. (2014). Regulations of P-glycoprotein/ABCB1/ MDR1 in human cancer cells. New J Sci, 2014, 1–10.
Kapse-Mistry, S., Govender, T., Srivastava, R., & Yergeri, M. (2014). Nanodrug delivery in reversing multidrug resistance in cancer cells. Frontiers in Pharmacology, 5, 1–22.
Thorn, C. F., Oshiro, C., Marsh, S., Hernandez-Boussard, T., McLeod, H., Klein, T. E., & Altman, R. B. (2011). Doxorubicin pathways. Pharmacogenetics and Genomics, 21, 440–446.
Patra, N., Dehury, N., Pal, A., Behera, A., & Patra, S. (2018). Preparation and mechanistic aspect of natural xanthone functionalized gold nanoparticle. Mater Sci Eng C, 90, 439–445.
Qin, J. L., Deng, S. P., Zhang, Y. L., Yuan, T., Li, Y. B., Han, H. H., Liu, Y. C., & Chen, Z. F. (2016). Water soluble copper (II) and zinc(II) complexes of mangiferin: Synthesis, antitumour activity and DNA binding studies. Journal of Chemical Research, 40, 659–663.
Akter, S., Moni, A., Faisal, G. M., Uddin, M. R., Jahan, N., Hannan, M. A., Rahman, A., & Uddin, M. J. (2022). Renoprotective effects of mangiferin: Pharmacological advances and future perspectives. Int. J. Environ. Res. Public Health, 19(9), 1864.
Sahu, A. K., Verma, V. K., Mutneja, E., Malik, S., Nag, T. C., Dinda, A. K., Arya, D. S., & Bhatia, J. (2019). Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of MAPK pathway. Molecular and Cellular Biochemistry, 452, 141–152.
Sadhukhan, P., Saha, S., Dutta, S., & Sil, P. C. (2018). Mangiferin ameliorates cisplatin induced acute kidney injury by upregulating Nrf-2 via the activation of PI3K and exhibits synergistic anticancer activity with cisplatin. Frontiers in Pharmacology, 9, 1–19.
Song, Y., Liu, W., Tang, K., Zang, J., Li, D., & Gao, H. (2020). Mangiferin alleviates renal interstitial fibrosis in streptozotocin-induced diabetic mice through regulating the PTEN/PI3K/Akt signaling pathway. J. Diabetes Research, 1, 1–12.
Saha, S., Sadhukhan, P., Sil, P. C., & Mangiferin. (2016). A xanthonoid with multipotent anti-inflammatory potential. Biofactors, 42, 459–474.
Rajendran, P., Rengarajan, T., Nishigaki, Y., Palaniswami, R., & Nishigaki, I. (2016). In vitro studies on mangiferin protection against cadmium-induced human renal endothelial damage and cell death via the MAP kinase and NF-B pathways. J Recept Signal Transduct, 36, 57–66.
He, L., Peng, X., Zhu, J., Chen, X., Liu, H., Tang, C., Dong, Z., Liu, F., & Peng, Y. (2014). Mangiferin attenuate sepsis-induced acute kidney injury via antioxidant and anti-inammatory effects. American Journal of Nephrology, 40, 441–450.
Yang, H., Gao, L., Niu, Y., Zhou, Y., Lin, H., Jiang, J., Kong, X., Liu, X., & Li, L. (2015). Mangiferin inhibits renal urate reabsorption by modulating urate transporters in experimental hyperuricemia. Biological &/And Pharmaceutical Bulletin, 38, 1591–1598.
Pal, P. B., Sinha, K., & Sil, P. C. (2014). Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PLoS One, 9(9), 10720.
Owczarek, K., & Jaworski, M. (2016). Quality of life and severity of skin changes in the dynamics of psoriasis. Postep Dermatologii i Alergol, 33, 102–108.
Moy, L., Lake, E. P., & Swan, J. (2018). Review of the efficacy and safety of topical Mahonia aquifolium for the treatment of psoriasis and atopic dermatitis. J Clin Aesthet Dermatol, 11, 42–47.
Gonzalez-Mira, E., Egea, M. A., Garcia, M. L., & Souto, E. B. (2010). Colloids and surfaces B: Biointerfaces design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surfaces B Biointerfaces, 81, 412–421.
Beebe, D. C., Holekamp, M., & Shui, Y. (2010). Oxidative damage and the prevention of age-related cataracts. Ophthalmic Research, 44(3), 155–165.
Kim, S. J., Sung, M. S., Heo, H., Lee, J. H., & Park, S. W. (2016). Mangiferin protects retinal ganglion cells in ischemic mouse retina via SIRT1. Current Eye Research, 41(6), 844–855.
Valles, E. G., de Castro, C. R., & Castro, J. A. (1994). N-Acetyl cysteine is an early but also a late preventive agent against carbon tetrachloride-induced liver necrosis. Toxicology Letters, 71, 87–95.
Recknagel, R. O., Glende, E. A., Dolak, J. A., & Waller, R. L. (1989). Mechanisms of carbon tetrachloride toxicity. Pharmacology & Therapeutics, 43, 139–154.
Roy, R., Chakraborty, A., Jana, K., Sarkar, B., Biswas, P., & Madhu, N. R. (2023). The broader aspects of treating diabetes with the application of nanobiotechnology. In R. Noor (Ed.), Advances in diabetes research and management. Springer.
Sekar, V., Mani, S., Malarvizhi, R., Nithya, P., & Vasanthi, H. R. (2019). Antidiabetic effect of mangiferin in combination with oral hypoglycemic agents’ metformin and gliclazide. Phytomedicine, 59, 152901.
Prabhu, S., Punithavathi, A., & Sandhya, V. (2021). S. C. Atul, entrapment of mangiferin polyphenol in poly lactic-co-glycolic acid nanoparticles for treatment of lung cancer. WO201941036882.
Katti, K., Khhobchandani, M., Khan, A., et al. (2018). Univ Missouri. Ayurvedic encapsulated gold nanoparticles, fabrication methods and cancer therapeutic methods. WO2018152002.
Katti, K., Cutler, C., & Khoobchandani, M. (2017). Univ Missouri. Mangiferin encapsulated gold nanoparticles, fabrication methods and cancer therapeutic methods. WO2018067570.
Zhang, J., Lei, T., Zhao, H., et al. (2016). Univ Chongqing Medical. Oil-in-water nano-emulsion capable of obviously improving bioavailability of insoluble medicament and preparation method for oil-in-water nano-emulsion. CN106038488.
Zhang, J., Chen, J., Jiang, X., et al. (2016). Univ Chongqing Medical. Water-inoil type nano-emulsion capable of obviously improving bioavailability of poorly water-soluble drugs and preparation method of water-in-oil type nano-emulsion. CN105997875.
Deng, J., Huang, J., Li, X., et al. (2014). Univ Guangxi traditional Chinese medical. Multi-element mangiferin solid dispersion as well as preparation method and application thereof. CN1044738.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participation
Not applicable.
Consent for Publication
All the authors had their consent for the publication of the reported study. Conceptualisation: H.B. and M.B.; writing—original draft preparation: M.B., P.K., I.B., and M.B.; writing—review and editing: K.S., G.S., and M.B.; supervision: H.B. and G.J. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baghel, M., Baghel, I., Kumari, P. et al. Nano-delivery Systems and Therapeutic Applications of Phytodrug Mangiferin. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-04906-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-024-04906-6