Abstract
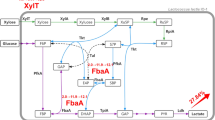
Xylitol is a polyol widely used in food, pharmaceuticals, and light industries. It is currently produced through the chemical catalytic hydrogenation of xylose and generates xylose mother liquor as a substantial byproduct in the procedure of xylose extraction. If xylose mother liquor could also be efficiently bioconverted to xylitol, the greenness and atom economy of xylitol production would be largely improved. However, xylose mother liquor contains a mixture of glucose, xylose, and arabinose, raising the issue of carbon catabolic repression in its utilization by microbial conversion. Targeting this challenge, the transcriptional activator XylR was overexpressed in a previously constructed xylitol-producing E. coli strain CPH. The resulting strain CPHR produced 16.61 g/L of xylitol in shake-flask cultures from the mixture of corn cob hydrolysate and xylose mother liquor (1:1, v/v) with a xylose conversion rate of 90.1%, which were 2.23 and 2.15 times higher than the starting strain, respectively. Furthermore, XylR overexpression upregulated the expression levels of xylE, xylF, xylG, and xylH genes by 2.08–2.72 times in arabinose-containing medium, suggesting the alleviation of transcriptional repression of xylose transport genes by arabinose. This work lays the foundation for xylitol bioproduction from xylose mother liquor.





Similar content being viewed by others
Data Availability
Original data generated in this study are available from the corresponding author on reasonable request.
Abbreviations
- HPLC:
-
High-Performance Liquid Chromatography
- PCR:
-
Polymerase Chain Reaction
- GFP:
-
Green Fluorescent Protein
- CCR:
-
Carbon Catabolite Repression
- CRP:
-
Cyclic AMP Receptor Protein
References
Bozell, J. J., & Petersen, G. R. (2010). Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “top 10” revisited. Green Chemistry, 12(4), 539–555. https://doi.org/10.1039/b922014c
Wei, J., Yuan, Q., Wang, T., & Wang, L. (2010). Purification and crystallization of xylitol from fermentation broth of corncob hydrolysates. Frontiers of Chemical Engineering in China, 4(1), 57–64. https://doi.org/10.1007/s11705-009-0295-1
Delgado Arcaño, Y., Valmaña García, O. D., Mandelli, D., Carvalho, W. A., & Magalhães Pontes, L. A. (2020). Xylitol: A review on the progress and challenges of its production by chemical route. Catalysis Today, 344, 2–14. https://doi.org/10.1016/j.cattod.2018.07.060
Barathikannan, K., & Agastian, P. (2016). Xylitol: Production, optimization and industrial application. International Journal of Current Microbiology and Applied Sciences, 5(9), 324–339. https://doi.org/10.20546/ijcmas.2016.509.036
Wang, H., Li, L., Zhang, L., An, J., Cheng, H., & Deng, Z. (2016). Xylitol production from waste xylose mother liquor containing miscellaneous sugars and inhibitors: One-pot biotransformation by Candida tropicalis and recombinant Bacillus subtilis. Microbial Cell Factories, 15(1), 1–12. https://doi.org/10.1186/s12934-016-0480-0
Swernath, S., Kaspereit, M., & Kienle, A. (2013). Dynamics and control of coupled continuous chromatography and crystallization processes for the production of pure enantiomers. Chemical Engineering and Technology, 36(8), 1417–1429. https://doi.org/10.1002/ceat.201200279
Görke, B., & Stülke, J. (2008). Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nature Reviews Microbiology, 6(8), 613–624. https://doi.org/10.1038/nrmicro1932
Yuan, D., Liu, B., Yuan, X., Feng, L., Xu, X., Zhu, J., … Wu, M. (2023). Multisite mutation of the Escherichia coli cAMP receptor protein: Enhancing xylitol biosynthesis by activating xylose catabolism and improving strain tolerance. Journal of Agricultural and Food Chemistry, acs.jafc.3c05445. https://doi.org/10.1021/acs.jafc.3c05445
Denisov, V. P., & Halle, B. (1998). Thermal denaturation of ribonuclease A characterized by water 17 O and 2 H magnetic relaxation dispersion. Biochemistry, 37(26), 9595–9604. https://doi.org/10.1021/bi980442b
Khankal, R., Chin, J. W., & Cirino, P. C. (2008). Role of xylose transporters in xylitol production from engineered Escherichia coli. Journal of Biotechnology, 134(3–4), 246–252. https://doi.org/10.1016/j.jbiotec.2008.02.003
Griffith, J. K., Baker, M. E., Rouch, D. A., Page, M. G. P., Skurray, R. A., Paulsen, I. T., & Henderson, P. J. F. (1992). Membrane transport proteins: implications of sequence comparisons. Current Opinion in Cell Biology, 4(4), 684–695. https://doi.org/10.1016/0955-0674(92)90090-Y
Ahlem, C., Huisman, W., Neslund, G., & Dahms, A. S. (1982). Purification and properties of a periplasmic D-xylose-binding protein from Escherichia coli K-12. Journal of Biological Chemistry, 257(6), 2926–2931. https://doi.org/10.1016/S0021-9258(19)81053-0
Desai, T. A., & Rao, C. V. (2010). Regulation of arabinose and xylose metabolism in Escherichia coli. Applied and Environmental Microbiology, 76(5), 1524–1532. https://doi.org/10.1128/AEM.01970-09
Koirala, S., Wang, X., & Rao, C. V. (2016). Reciprocal regulation of L-arabinose and D-xylose metabolism in Escherichia coli. Journal of Bacteriology, 198(3), 386–393. https://doi.org/10.1128/JB.00709-15
Yuan, X., Tu, S., Lin, J., Yang, L., Shen, H., & Wu, M. (2020). Combination of the CRP mutation and ptsG deletion in Escherichia coli to efficiently synthesize xylitol from corncob hydrolysates. Applied Microbiology and Biotechnology, 104(5), 2039–2050. https://doi.org/10.1007/s00253-019-10324-0
Su, B., Wu, M., Zhang, Z., Lin, J., & Yang, L. (2015). Efficient production of xylitol from hemicellulosic hydrolysate using engineered Escherichia coli. Metabolic Engineering, 31, 112–122. https://doi.org/10.1016/j.ymben.2015.07.003
Lu, C., Bentley, W. E., & Rao, G. (2004). A high-throughput approach to promoter study using green fluorescent protein. Biotechnology Progress, 20(6), 1634–1640. https://doi.org/10.1021/bp049751l
Liang, T., Sun, J., Ju, S., Su, S., Yang, L., & Wu, J. (2021). Construction of T7-like expression system in pseudomonas putida KT2440 to enhance the heterologous expression level. Frontiers in Chemistry, 9, 664967. https://doi.org/10.3389/fchem.2021.664967
Deutscher, J. (2008). The mechanisms of carbon catabolite repression in bacteria. Current Opinion in Microbiology, 11(2), 87–93. https://doi.org/10.1016/j.mib.2008.02.007
Gosset, G. (2005). Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate:Sugar phosphotransferase system. Microbial Cell Factories, 4(1), 14–24. https://doi.org/10.1186/1475-2859-4-14
Hernández-Montalvo, V., Martínez, A., Hernández-Chavez, G., Bolivar, F., Valle, F., & Gosset, G. (2003). Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnology and Bioengineering, 83(6), 687–694. https://doi.org/10.1002/bit.10702
Chiang, C. J., Lee, H. M., Guo, H. J., Wang, Z. W., Lin, L. J., & Chao, Y. P. (2013). Systematic approach to engineer escherichia coli pathways for co-utilization of a glucose-xylose mixture. Journal of Agricultural and Food Chemistry, 61(31), 7583–7590. https://doi.org/10.1021/jf401230r
Guo, Q., Ullah, I., Zheng, L. J., Gao, X. Q., Liu, C. Y., Zheng, H. D., & Deng, L. (2022). Intelligent self-control of carbon metabolic flux in SecY-engineered Escherichia coli for xylitol biosynthesis from xylose-glucose mixtures. Biotechnology and Bioengineering, 119(2), 388–398. https://doi.org/10.1002/bit.28002
Groff, D., Benke, P. I., Batth, T. S., Bokinsky, G., Petzold, C. J., Adams, P. D., & Keasling, J. D. (2012). Supplementation of intracellular XylR leads to coutilization of hemicellulose sugars. Applied and Environmental Microbiology, 78(7), 2221–2229. https://doi.org/10.1128/AEM.06761-11
Sievert, C., Nieves, L. M., Panyon, L. A., Loeffler, T., Morris, C., Cartwright, R. A., & Demain, A. L. (2017). Experimental evolution reveals an effective avenue to release catabolite repression via mutations in XylR. Proceedings of the National Academy of Sciences of the United States of America, 114(28), 7349–7354. https://doi.org/10.1073/pnas.1700345114
Sha, F., Zheng, Y., Chen, J., Chen, K., Cao, F., Yan, M., & Ouyang, P. (2018). D-Tagatose manufacture through bio-oxidation of galactitol derived from waste xylose mother liquor. Green Chemistry, 20(10), 2382–2391. https://doi.org/10.1039/c8gc00091c
Zhang, L., Chen, Z., Wang, J., Shen, W., Li, Q., & Chen, X. (2021). Stepwise metabolic engineering of Candida tropicalis for efficient xylitol production from xylose mother liquor. Microbial Cell Factories, 20(1), 105–116. https://doi.org/10.1186/s12934-021-01596-1
Wang, H., Pan, J., Wang, J., Wang, N., Zhang, J., Li, Q., & Zhou, X. (2014). Review; agriculture and environmental biotechnology succinic acid production from xylose mother liquor by recombinant Escherichia coli strain. Biotechnology and Biotechnological Equipment, 28(6), 1042–1049. https://doi.org/10.1080/13102818.2014.952501
Li, Y., Zhang, H., Zhu, J., Yan, Q, Wang, Q., & Naikun, S. (n.d.). Optimization of succinic acid fermentation from xylose mother liquor by response surface methodology. https://doi.org/10.16085/j.issn.1000-6613.2017-0631
Feng, J., Li, T., Zhang, X., Chen, J., Zhao, T., & Zou, X. (2019). Efficient production of polymalic acid from xylose mother liquor, an environmental waste from the xylitol industry, by a T-DNA-based mutant of Aureobasidium pullulans. Applied Microbiology and Biotechnology, 103, 6519–6527. https://doi.org/10.1007/s00253-019-09974-x
Sakakibara, Y., Saha, B. C., & Taylor, P. (2009). Microbial production of xylitol from l-arabinose by metabolically engineered Escherichia coli. Journal of Bioscience and Bioengineering, 107(5), 506–511. https://doi.org/10.1016/j.jbiosc.2008.12.017
Funding
This work was supported by the National Natural Science Foundation of China (no. 22278362) and the Public Welfare Project of Zhejiang Provincial Science and Technology Department (no. LGG21B06005).
Author information
Authors and Affiliations
Contributions
Investigation and validation: Bingbing Liu, Lin Jiang, and Gang Xu. Administrative support: Mianbin Wu, Lidan Ye, and Lirong Yang. Methodology and data analysis: Dongxu Yuan, Jianping Lin, and Yuhuan Chen. Manuscript writing: Dongxu Yuan and Bingbing Liu. Review and editing: Mianbin Wu, Lidan Ye, Yiqi Jiang, and Jizhang Lian. Final approval of manuscript: all authors.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
All authors consent to participate in the project.
Consent for Publication
All authors consent to publish the paper.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, D., Liu, B., Jiang, L. et al. XylR Overexpression in Escherichia coli Alleviated Transcriptional Repression by Arabinose and Enhanced Xylitol Bioproduction from Xylose Mother Liquor. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-04890-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-024-04890-x