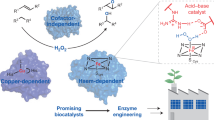
Abstract
Peroxdiase is one of the member of oxireductase super family, which has a broad substrate range and a variety of reaction types, including hydroxylation, epoxidation or halogenation of unactivated C-H bonds, and aromatic group or biophenol compounds. Here, we summarized the recently discovered enzymes with peroxidation activity, and focused on the special structures, sites, and corresponding strategies that can change the peroxidase catalytic activity, stability, and substrate range. The comparison of the structural differences between these natural enzymes and the mimic enzymes of binding nanomaterials and polymer materials is helpful to expand the application of peroxidase in industry. In addition, we also reviewed the catalytic application of peroxidase in the synthesis of important organic molecules and the degradation of pollutants.



Similar content being viewed by others
References
Shen, C., Shan, T., Zhao, W., Ou, C., Li, L., Liu, X., Liu, J., & Yu, B. (2019). Regio- and enantioselective O-demethylation of tetrahydroprotoberberines by cytochrome P450 enzyme system from Streptomyces griseus ATCC 13273. Applied Microbiology and Biotechnology, 103, 761–776.
Mukherjee, M., & Dey, A. (2020). Catalytic C–H bond oxidation using dioxygen by analogues of heme superoxide. Inorganic Chemistry, 59, 7415–7425.
Di Nardo, G., & Gilardi, G. (2020). Natural compounds as pharmaceuticals: The key role of cytochromes P450 reactivity. Trends in Biochemical Sciences, 45, 511–525.
Hobisch, M., Holtmann, D., Gomez de Santos, P., Alcalde, M., Hollmann, F., & Kara, S. (2021). Recent developments in the use of peroxygenases – Exploring their high potential in selective oxyfunctionalisations. Biotechnology Advances, 51, 107615.
Sigmund, M.-C., & Poelarends, G. J. (2020). Current state and future perspectives of engineered and artificial peroxygenases for the oxyfunctionalization of organic molecules. Nature Catalysis, 3, 690–702.
Kwon, H., Basran, J., Devos, J. M., Suardíaz, R., van der Kamp, M. W., Mulholland, A. J., Schrader, T. E., Ostermann, A., Blakeley, M. P., & Moody, P. C. (2020). Visualizing the protons in a metalloenzyme electron proton transfer pathway. Proceedings of the National Academy of Sciences, 117, 6484–6490.
Stasyuk, N., Smutok, O., Demkiv, O., Prokopiv, T., Gayda, G., Nisnevitch, M., & Gonchar, M. (2020). Synthesis, catalytic properties and application in biosensorics of nanozymes and electronanocatalysts: A review. Sensors, 20, 4509.
Feng, L., Zhang, L., Zhang, S., Chen, X., Li, P., Gao, Y., Xie, S., Zhang, A., & Wang, H. (2020). Plasma-assisted controllable doping of nitrogen into MoS2 nanosheets as efficient nanozymes with enhanced peroxidase-like catalysis activity. ACS Applied Materials & Interfaces, 12, 17547–17556.
Baldim, V., Yadav, N., Bia, N., Graillot, A., Loubat, C., Singh, S., Karakoti, A. S., & Berret, J.-F. (2020). Polymer-coated cerium oxide nanoparticles as oxidoreductase-like catalysts. ACS Applied Materials & Interfaces, 12, 42056–42066.
Guo, Y., Xu, L., & Liu, A. (2020). Boosting the peroxidase-like activity of cobalt ions by amino acid-based biological species and its applications. Chemistry–An Asian Journal, 15, 1067–1073.
Pramanik, K., Sengupta, P., Majumder, B., Datta, P., & Sarkar, P. (2020). Artificial bifunctional photozyme of glucose oxidase–peroxidase for solar-powered glucose–peroxide detection in a biofluid with resorcinol–formaldehyde polymers. ACS Applied Materials & Interfaces, 12, 36948–36956.
Liu, Z., Xie, L., Qiu, K., Liao, X., Rees, T. W., Zhao, Z., Ji, L., & Chao, H. (2020). An ultrasmall RuO2 nanozyme exhibiting multienzyme-like activity for the prevention of acute kidney injury. ACS Applied Materials & Interfaces, 12, 31205–31216.
Jo, S.-M., Zhang, K. A. I., Wurm, F. R., & Landfester, K. (2020). Mimic of the cellular antioxidant defense system for a sustainable regeneration of nicotinamide adenine dinucleotide (NAD). ACS Applied Materials & Interfaces, 12, 25625–25632.
Mohamad, A., Rizwan, M., Keasberry, N. A., Nguyen, A. S., Lam, T. D., & Ahmed, M. U. (2020). Gold-microrods/Pd-nanoparticles/polyaniline-nanocomposite-interface as a peroxidase-mimic for sensitive detection of tropomyosin. Biosensors & Bioelectronics, 155, 112108.
Xie, Y., Xu, M., Wang, L., Liang, H., Wang, L., & Song, Y. (2020). Iron-porphyrin-based covalent-organic frameworks for electrochemical sensing H2O2 and pH. Materials Science and Engineering, C, 112, 110864.
Ye, R., Xu, H., Gu, J., & Chen, H. (2021). Bioinspired synthesis of protein-posnjakite organic-inorganic nanobiohybrid for biosensing applications. Analytica Chimica Acta, 1143, 31–36.
Zhang, X., King-Smith, E., & Renata, H. (2018). Total synthesis of Tambromycin by combining chemocatalytic and biocatalytic C− H functionalization. Angewandte Chemie, International Edition, 57, 5037–5041.
Kim, S. J., Joo, J. C., Song, B. K., Yoo, Y. J., & Kim, Y. H. (2016). Improving the synthesis of phenolic polymer using Coprinus cinereus peroxidase mutant Phe230Ala. Enzyme and Microbial Technology, 87-88, 37–43.
Rekik, H., Zaraî Jaouadi, N., Bouacem, K., Zenati, B., Kourdali, S., Badis, A., Annane, R., Bouanane-Darenfed, A., Bejar, S., & Jaouadi, B. (2019). Physical and enzymatic properties of a new manganese peroxidase from the white-rot fungus Trametes pubescens strain i8 for lignin biodegradation and textile-dyes biodecolorization. International Journal of Biological Macromolecules, 125, 514–525.
Granja-Travez, R. S., Wilkinson, R. C., Persinoti, G. F., Squina, F. M., Fülöp, V., & Bugg, T. D. H. (2018). Structural and functional characterisation of multi-copper oxidase CueO from lignin-degrading bacterium Ochrobactrum sp. reveal its activity towards lignin model compounds and lignosulfonate. FEBS Journal, 285, 1684–1700.
Linde, D., Olmedo, A., González-Benjumea, A., Estévez, M., Renau-Mínguez, C., Carro, J., Fernández-Fueyo, E., Gutiérrez, A., Martínez, A. T., & Master, E. R. (2020). Two new unspecific peroxygenases from heterologous expression of fungal genes in Escherichia coli. Applied and Environmental Microbiology, 86, e02899-19.
Girvan, H. M., Poddar, H., McLean, K. J., Nelson, D. R., Hollywood, K. A., Levy, C. W., Leys, D., & Munro, A. W. (2018). Structural and catalytic properties of the peroxygenase P450 enzyme CYP152K6 from Bacillus methanolicus. Journal of Inorganic Biochemistry, 188, 18–28.
Olmedo, A., Aranda, C., Del Río, J. C., Kiebist, J., Scheibner, K., Martínez, A. T., & Gutiérrez, A. (2016). From alkanes to carboxylic acids: Terminal oxygenation by a fungal peroxygenase. Angewandte Chemie, 128, 12436–12439.
Chowhan, R. K., Rahaman, H., & Singh, L. R. (2020). Structural basis of peroxidase catalytic cycle of human Prdx6. Scientific Reports, 10, 17416.
Lian, F.-M., Jiang, Y.-L., Yang, W., & Yang, X. (2020). Crystal structure of sulfonic peroxiredoxin Ahp1 in complex with thioredoxin Trx2 mimics a conformational intermediate during the catalytic cycle. International Journal of Biological Macromolecules, 161, 1055–1060.
Sun, C.-C., Dong, W.-R., Shao, T., Li, J.-Y., Zhao, J., Nie, L., Xiang, L.-X., Zhu, G., & Shao, J.-Z. (2017). Peroxiredoxin 1 (Prx1) is a dual-function enzyme by possessing Cys-independent catalase-like activity. The Biochemical Journal, 474, 1373–1394.
Yan, D., Wang, G., Xiong, F., Sun, W.-Y., Shi, Z., Lu, Y., Li, S., & Zhao, J. (2018). A selenium-catalysed para-amination of phenols. Nature Communications, 9, 4293.
Zhang, Y., Liu, H., Dai, X., Cai, C., Wang, J., Wang, M., Shen, Y., & Wang, P. (2020). Impact of application of heat-activated persulfate oxidation treated erythromycin fermentation residue as a soil amendment: Soil chemical properties and antibiotic resistance. The Science of the Total Environment, 736, 139668.
Fejzagić, A. V., Gebauer, J., Huwa, N., & Classen, T. (2019). Halogenating enzymes for active agent synthesis: First steps are done and many have to follow. Molecules, 24, 4008.
Wang, K., Huang, X., & Lin, K. (2019). Multiple catalytic roles of chloroperoxidase in the transformation of phenol: Products and pathways. Ecotoxicology and Environmental Safety, 179, 96–103.
Frank, A., Seel, C. J., Groll, M., & Gulder, T. (2016). Characterization of a cyanobacterial haloperoxidase and evaluation of its biocatalytic halogenation potential. ChemBioChem, 17, 2028–2032.
Li, F., & Tang, Y. (2021). The activation mechanism of peroxidase by ultrasound. Ultrasonics Sonochemistry, 71, 105362.
Wang, L., Wei, S., Pan, X., Liu, P., Du, X., Zhang, C., Pu, L., & Wang, Q. (2018). Enhanced turnover for the P450 119 peroxygenase-catalyzed asymmetric epoxidation of styrenes by random mutagenesis. Chemistry - A European Journal, 24, 2741–2749.
Molina-Espeja, P., Cañellas, M., Plou, F. J., Hofrichter, M., Lucas, F., Guallar, V., & Alcalde, M. (2016). Synthesis of 1-naphthol by a natural peroxygenase engineered by directed evolution. ChemBioChem, 17, 341–349.
Ramirez-Ramirez, J., Martin-Diaz, J., Pastor, N., Alcalde, M., & Ayala, M. (2020). Exploring the role of phenylalanine residues in modulating the flexibility and topography of the active site in the peroxygenase variant PaDa-I. International Journal of Molecular Sciences, 21, 5734.
Ma, N., Chen, Z., Chen, J., Chen, J., Wang, C., Zhou, H., Yao, L., Shoji, O., Watanabe, Y., & Cong, Z. (2018). Dual-functional small molecules for generating an efficient cytochrome P450BM3 peroxygenase. Angewandte Chemie, 130, 7754–7759.
Humer, D., & Spadiut, O. (2019). Improving the performance of horseradish peroxidase by site-directed mutagenesis. International Journal of Molecular Sciences, 20, 916.
Sánchez-Alejandro, F., Baratto, M. C., Basosi, R., Graeve, O., & Vazquez-Duhalt, R. (2019). Addition of new catalytic sites on the surface of versatile peroxidase for enhancement of LRET catalysis. Enzyme and Microbial Technology, 131, 109429.
Fujieda, N., Nakano, T., Taniguchi, Y., Ichihashi, H., Sugimoto, H., Morimoto, Y., Nishikawa, Y., Kurisu, G., & Itoh, S. (2017). A well-defined osmium–cupin complex: Hyperstable artificial osmium peroxygenase. Journal of the American Chemical Society, 139, 5149–5155.
Yang, Y., Cho, I., Qi, X., Liu, P., & Arnold, F. H. (2019). An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp3)–H bonds. Nature Chemistry, 11, 987–993.
Ren, X., Liu, N., Chandgude, A. L., & Fasan, R. (2020). An enzymatic platform for the highly enantioselective and stereodivergent construction of cyclopropyl-δ-lactones. Angewandte Chemie, International Edition, 59, 21634–21639.
Cao, X., Liang, J., Aluko, R. E., & Thiyam-Holländer, U. (2019). In situ oxidation of canola meal sinapic acid by horseradish peroxidase (type II) and tyrosinase. Journal of Food Biochemistry, 43, e12884.
Sitte, E., & Senge, M. O. (2020). The red color of life transformed - Synthetic advances and emerging applications of protoporphyrin IX in chemical biology. European Journal of Organic Chemistry, 2020, 3171–3191.
Stenner, R., Steventon, J. W., Seddon, A., & Anderson, J. R. (2020). A de novo peroxidase is also a promiscuous yet stereoselective carbene transferase. Proceedings of the National Academy of Sciences, 117, 1419–1428.
Mirts, E. N., Petrik, I. D., Hosseinzadeh, P., Nilges, M. J., & Lu, Y. (2018). A designed heme-[4Fe-4S] metalloenzyme catalyzes sulfite reduction like the native enzyme. Science, 361, 1098–1101.
Caserta, G., Chino, M., Firpo, V., Zambrano, G., Leone, L., D’Alonzo, D., Nastri, F., Maglio, O., Pavone, V., & Lombardi, A. (2018). Enhancement of peroxidase activity in artificial mimochrome VI catalysts through rational design. ChemBioChem, 19, 1823–1826.
Ibrahim, H., Mulyk, P., & Sen, D. (2019). DNA G-quadruplexes activate heme for robust catalysis of carbene transfer reactions. ACS Omega, 4, 15280–15288.
Wang, Z. G., Li, Y., Wang, H., Wan, K., Liu, Q., Shi, X., & Ding, B. (2019). Enzyme mimic based on a self-assembled chitosan/DNA hybrid exhibits superior activity and tolerance. Chemistry - A European Journal, 25, 12576–12582.
Harraz, D. M., & Davis, J. T. (2018). A self-assembled peroxidase from 5′-GMP and heme. Chemical Communications, 54, 1587–1590.
Leone, L., Chino, M., Nastri, F., Maglio, O., Pavone, V., & Lombardi, A. (2020). Mimochrome, a metalloporphyrin-based catalytic Swiss knife†. Biotechnology and Applied Biochemistry, 67, 495–515.
Koebke, K. J., Kühl, T., Lojou, E., Demeler, B., Schoepp-Cothenet, B., Iranzo, O., Pecoraro, V. L., & Ivancich, A. (2020). The pH-induced selectivity between cysteine or histidine coordinated heme in an artificial α-helical metalloprotein. Angewandte Chemie, International Edition, 60, 3974–3978.
Bilal, M., Ashraf, S. S., Ferreira, L. F. R., Cui, J., Lou, W.-Y., Franco, M., & Iqbal, H. M. N. (2020). Nanostructured materials as a host matrix to develop robust peroxidases-based nanobiocatalytic systems. International Journal of Biological Macromolecules, 162, 1906–1923.
Zhang, L., Li, S., Dong, M., Jiang, Y., Li, R., Zhang, S., Lv, X., Chen, L., & Wang, H. (2017). Reconstituting redox active centers of heme-containing proteins with biomineralized gold toward peroxidase mimics with strong intrinsic catalysis and electrocatalysis for H2O2 detection. Biosensors & Bioelectronics, 87, 1036–1043.
Zhang, W., Burek, B. O., Fernández-Fueyo, E., Alcalde, M., Bloh, J. Z., & Hollmann, F. (2017). Selective activation of C−H bonds in a cascade process combining photochemistry and biocatalysis. Angewandte Chemie, International Edition, 56, 15451–15455.
Feyza Özgen, F., Runda, M. E., Burek, B. O., Wied, P., Bloh, J. Z., Kourist, R., & Schmidt, S. (2020). Artificial light-harvesting complexes enable Rieske oxygenase catalyzed hydroxylations in non-photosynthetic cells. Angewandte Chemie, International Edition, 59, 3982–3987.
Cai, S., & Yang, R. (2020). Two-dimensional nanomaterials with enzyme-like properties for biomedical applications. Frontiers in Chemistry, 8, 565940.
Bilal, M., Barceló, D., & Iqbal, H. M. N. (2020). Nanostructured materials for harnessing the power of horseradish peroxidase for tailored environmental applications. Science of the Total Environment, 749, 142360.
Yang, S., Yang, J., Wang, T., Li, L., Yu, S., Jia, R., & Chen, P. (2020). Construction of a combined enzyme system of graphene oxide and manganese peroxidase for efficient oxidation of aromatic compounds. Nanoscale, 12, 7976–7985.
Xi, Z., Gao, W., & Xia, X. (2020). Size effect in Pd−Ir core-shell nanoparticles as nanozymes. ChemBioChem, 21, 2440–2444.
Liu, D., Wang, D., Jing, X., Zhao, X., Xi, D., Dang, D., & Meng, L. (2020). Continuous phase regulation of MoSe2 from 2H to 1T for the optimization of peroxidase-like catalysis. Journal of Materials Chemistry, 8, 6451–6458.
Li, M., Chen, J., Wu, W., Fang, Y., & Dong, S. (2020). Oxidase-like MOF-818 nanozyme with high specificity for catalysis of catechol oxidation. Journal of the American Chemical Society, 142, 15569–15574.
Kariyawasam, K., Di Meo, T., Hammerer, F., Valerio-Lepiniec, M., Sciortino, G., Maréchal, J. D., Minard, P., Mahy, J. P., Urvoas, A., & Ricoux, R. (2020). An artificial hemoprotein with inducible peroxidase- and monooxygenase-like activities. Chemistry - A European Journal, 26, 14929–14937.
Ricco, R., Wied, P., Nidetzky, B., Amenitsch, H., & Falcaro, P. (2020). Magnetically responsive horseradish peroxidase@ ZIF-8 for biocatalysis. Chemical Communications, 56, 5775–5778.
Wang, D., Chai, Y., Yuan, Y., & Yuan, R. (2019). Lattice-like DNA tetrahedron nanostructure as scaffold to locate GOx and HRP enzymes for highly efficient enzyme cascade reaction. ACS Applied Materials & Interfaces, 12, 2871–2877.
Özacar, M., Mehde, A. A., Mehdi, W. A., Özacar, Z. Z., & Severgün, O. (2019). The novel multi cross-linked enzyme aggregates of protease, lipase, and catalase production from the sunflower seeds, characterization and application. Colloids and Surfaces. B, Biointerfaces, 173, 58–68.
Sun, D., Liu, X., Zhu, M., Chen, Y., Li, C., Cheng, X., Zhu, Z., Lu, F., & Qin, H.-M. (2019). Efficient biosynthesis of high-value succinic acid and 5-hydroxyleucine using a multienzyme cascade and whole-cell catalysis. Journal of Agricultural and Food Chemistry, 67, 12502–12510.
Dennig, A., Gandomkar, S., Cigan, E., Reiter, T. C., Haas, T., Hall, M., & Faber, K. (2018). Enantioselective biocatalytic formal α-amination of hexanoic acid to l-norleucine. Organic & Biomolecular Chemistry, 16, 8030–8033.
Chen, Y., Chu, H., Liu, W., & Feng, W. (2019). Simultaneous synthesis of l-DOPA and oxidation of d-amino acid by specific coupling of a peroxidase to d-amino acid oxidase. Enzyme and Microbial Technology, 121, 8–16.
Liu, Q., Ma, X., Cheng, H., Xu, N., Liu, J., & Ma, Y. (2017). Co-expression of l-glutamate oxidase and catalase in Escherichia coli to produce α-ketoglutaric acid by whole-cell biocatalyst. Biotechnology Letters, 39, 913–919.
Yang, J., Liang, J., Shao, L., Liu, L., Gao, K., Zhang, J.-L., Sun, Z., Xu, W., Lin, P., Yu, R., & Zi, J. (2020). Green production of silybin and isosilybin by merging metabolic engineering approaches and enzymatic catalysis. Metabolic Engineering, 59, 44–52.
Chuaboon, L., Wongnate, T., Punthong, P., Kiattisewee, C., Lawan, N., Hsu, C. Y., Lin, C. H., Bornscheuer, U. T., & Chaiyen, P. (2019). One-pot bioconversion of l-arabinose to l-ribulose in an enzymatic cascade. Angewandte Chemie, International Edition, 58, 2428–2432.
Gandomkar, S., Dennig, A., Dordic, A., Hammerer, L., Pickl, M., Haas, T., Hall, M., & Faber, K. (2018). Biocatalytic oxidative cascade for the conversion of fatty acids into α-ketoacids via internal H2O2 recycling. Angewandte Chemie, International Edition, 57, 427–430.
Matthews, S., Tee, K. L., Rattray, N. J., McLean, K. J., Leys, D., Parker, D. A., Blankley, R. T., & Munro, A. W. (2017). Production of alkenes and novel secondary products by P450 OleTJE using novel H2O2-generating fusion protein systems. FEBS Letters, 591, 737–750.
Jiang, Y., Li, Z., Zheng, S., Xu, H., Zhou, Y. J., Gao, Z., Meng, C., & Li, S. (2020). Establishing an enzyme cascade for one-pot production of α-olefins from low-cost triglycerides and oils without exogenous H2O2 addition. Biotechnology for Biofuels, 13, 52.
Carro, J., Fernández-Fueyo, E., Fernández-Alonso, C., Cañada, J., Ullrich, R., Hofrichter, M., Alcalde, M., Ferreira, P., & Martínez, A. T. (2018). Self-sustained enzymatic cascade for the production of 2,5-furandicarboxylic acid from 5-methoxymethylfurfural. Biotechnology for Biofuels, 11, 86.
Jia, H. Y., Zong, M. H., Zheng, G. W., & Li, N. (2019). One-pot enzyme cascade for controlled synthesis of furancarboxylic acids from 5-hydroxymethylfurfural by H2O2 internal recycling. ChemSusChem, 12, 4764–4768.
Li, R., Zhou, X., Liu, D., & Feng, W. (2018). Enhancing the activity and stability of Mn-superoxide dismutase by one-by-one ligation to catalase. Free Radical Biology & Medicine, 129, 138–145.
Liu, Z., Lv, Y., & An, Z. (2017). Enzymatic cascade catalysis for the synthesis of multiblock and ultrahigh-molecular-weight polymers with oxygen tolerance. Angewandte Chemie, 129, 14040–14044.
Zwick, C., Sosa, M., & Renata, H. (2019). Characterization of a citrulline 4-hydroxylase from Nonribosomal Peptide GE81112 biosynthesis and engineering of its substrate specificity for the chemoenzymatic synthesis of enduracididine. Angewandte Chemie (International Ed. in English), 58, 18854–18858.
Shrivas, P., Zodape, S., Wankhade, A., & Pratap, U. (2020). Facile synthesis of benzazoles through biocatalytic cyclization and dehydrogenation employing catalase in water. Enzyme and Microbial Technology, 138, 109562.
Zhang, J.-Q., Li, G.-P., Kang, Y.-L., Teng, B.-H., & Yao, C.-S. (2017). Biomimetic synthesis of resveratrol trimers catalyzed by horseradish peroxidase. Molecules, 22, 819.
Hammer, A. K., Albrecht, F., Hahne, F., Jordan, P., Fraatz, M. A., Ley, J., Geissler, T., Schrader, J., Zorn, H., & Buchhaupt, M. (2020). Biotechnological production of odor-active methyl-branched aldehydes by a novel α-dioxygenase from Crocosphaera subtropica. Journal of Agricultural and Food Chemistry, 68, 10432–10440.
Levin, G., Gómez, S., Glodowsky, A., Cascone, O., & Hernáiz, M. J. (2018). Two-step enzymatic strategy for the synthesis of a smart phenolic polymer and further immobilization of a β-galactosidase able to catalyze transglycosydation reaction. International Journal of Biological Macromolecules, 117, 264–270.
Vanella, R., Ta, D. T., & Nash, M. A. (2019). Enzyme-mediated hydrogel encapsulation of single cells for high-throughput screening and directed evolution of oxidoreductases. Biotechnology and Bioengineering, 116, 1878–1886.
Fetherolf, M. M., Levy-Booth, D. J., Navas, L. E., Liu, J., Grigg, J. C., Wilson, A., Katahira, R., Beckham, G. T., Mohn, W. W., & Eltis, L. D. (2020). Characterization of alkylguaiacol-degrading cytochromes P450 for the biocatalytic valorization of lignin. Proceedings of the National Academy of Sciences, 117, 25771–25778.
Ion, S. G., Brudiu, T., Hanganu, A., Munteanu, F., Enache, M., Maria, G.-M., Tudorache, M., & Parvulescu, V. (2020). Biocatalytic strategy for grafting natural lignin with aniline. Molecules, 25, 4921.
Wang, C., Ouyang, X., Su, S., Liang, X., Zhang, C., Wang, W., Yuan, Q., & Li, Q. (2016). Effect of sulfonated lignin on enzymatic activity of the ligninolytic enzymes Cα-dehydrogenase LigD and β-etherase LigF. Enzyme and Microbial Technology, 93-94, 59–69.
Wang, X., Yao, B., & Su, X. (2018). Linking enzymatic oxidative degradation of lignin to organics detoxification. International Journal of Molecular Sciences, 19, 3373.
Catucci, G., Valetti, F., Sadeghi, S. J., & Gilardi, G. (2020). Biochemical features of dye-decolorizing peroxidases: Current impact on lignin degradation. Biotechnology and Applied Biochemistry, 67, 751–759.
Lin, L., Wang, X., Cao, L., & Xu, M. (2019). Lignin catabolic pathways reveal unique characteristics of dye-decolorizing peroxidases inPseudomonas putida. Environmental Microbiology, 21, 1847–1863.
Moural, T. W., Lewis, K. M., Barnaba, C., Zhu, F., Palmer, N. A., Sarath, G., Scully, E. D., Jones, J. P., Sattler, S. E., & Kang, C. (2017). Characterization of class III peroxidases from Switchgrass. Plant Physiology, 173, 417–433.
Ion, S., Opris, C., Cojocaru, B., Tudorache, M., Zgura, I., Galca, A. C., Bodescu, A. M., Enache, M., Maria, G.-M., & Parvulescu, V. I. (2018). One-pot enzymatic production of lignin-composites. Frontiers in Chemistry, 6, 124.
Fall, I., Czerwiec, Q., Abdellaoui, S., Doumeche, B., Ochs, M., Remond, C., & Rakotoarivonina, H. (2023). A thermostable bacterial catalase-peroxidase oxidizes phenolic compounds derived from lignins. Applied Microbiology and Biotechnology, 107, 201–217.
Ilić Đurđić, K., Ostafe, R., Đurđević Đelmaš, A., Popović, N., Schillberg, S., Fischer, R., & Prodanović, R. (2020). Saturation mutagenesis to improve the degradation of azo dyes by versatile peroxidase and application in form of VP-coated yeast cell walls. Enzyme and Microbial Technology, 136, 109509.
Krahe, N.-K., Berger, R. G., & Ersoy, F. (2020). A DyP-type peroxidase of Pleurotus sapidus with alkene cleaving activity. Molecules, 25, 1536.
Sung, H. J., Khan, M. F., & Kim, Y. H. (2019). Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. International Journal of Biological Macromolecules, 136, 20–26.
Chino, M., La Gatta, S., Leone, L., De Fenza, M., Lombardi, A., Pavone, V., & Maglio, O. (2023). Dye decolorization by a miniaturized peroxidase Fe-mimochromeVI*a. International Journal of Molecular Sciences, 24, 11070.
Bilal, M., Barceló, D., & Iqbal, H. M. N. (2020). Persistence, ecological risks, and oxidoreductases-assisted biocatalytic removal of triclosan from the aquatic environment. Science of the Total Environment, 735, 139194.
Xie, P., Fan, L., Huang, L., & Zhang, C. (2021). Oxidative polymerization of hydroxytyrosol catalyzed by laccase, tyrosinase or horseradish peroxidase: influencing factors and molecular simulations. Journal of Biomolecular Structure & Dynamics, 39, 5486–5497.
Bilal, M., Singh, A. K., Iqbal, H. M. N., Kim, T. H., Boczkaj, G., Athmaneh, K., & Ashraf, S. S. (2023). Bio-mitigation of organic pollutants using horseradish peroxidase as a promising biocatalytic platform for environmental sustainability. Environmental Research, 239, 117192.
Leng, Y., Bao, J., Xiao, H., Song, D., Du, J., Mohapatra, S., Werner, D., & Wang, J. (2020). Transformation mechanisms of tetracycline by horseradish peroxidase with/without redox mediator ABTS for variable water chemistry. Chemosphere, 258, 127306.
Yao, Y., & Li, Q. X. (2023). Efficient, fast and robust degradation of chlortetracycline in wastewater catalyzed by recombinant Arthromyces ramosus peroxidase. The Science of the Total Environment, 858, 159872.
Liu, X., Xue, P., Jia, F., Shi, K., Gu, Y., Ma, L., & Li, R. (2021). A novel approach to efficient degradation of indole using co-immobilized horseradish peroxidase-syringaldehyde as biocatalyst. Chemosphere, 262, 128411.
Cai, S., Chen, L.-W., Ai, Y.-C., Qiu, J.-G., Wang, C.-H., Shi, C., He, J., Cai, T.-M., & Liu, S.-J. (2017). Degradation of diphenyl ether in Sphingobium phenoxybenzoativorans SC_3 is initiated by a novel ring cleavage dioxygenase. Applied and Environmental Microbiology, 83, e00104–e00117.
Mukherjee, D., Bhattacharya, S., Taylor, K. E., & Biswas, N. (2019). Enzymatic treatment for removal of hazardous aqueous arylamines, 4,4′-methylenedianiline and 4,4′-thiodianiline. Chemosphere, 235, 365–372.
Kurapati, R., Mukherjee, S. P., Martín, C., Bepete, G., Vázquez, E., Pénicaud, A., Fadeel, B., & Bianco, A. (2018). Degradation of single-layer and few-layer graphene by neutrophil myeloperoxidase. Angewandte Chemie, International Edition, 57, 11722–11727.
Kurapati, R., Martìn, C., Palermo, V., Nishina, Y., & Bianco, A. (2021). Biodegradation of graphene materials catalyzed by human eosinophil peroxidase. Faraday Discussions, 227, 189–203.
Wang, J. M., Wang, C. M., Men, X., Yue, T. Q., Madzak, C., Xiang, X. H., Xiang, H. Y., & Zhang, H. B. (2020). Construction of arming Yarrowia lipolytica surface-displaying soybean seed coat peroxidase for use as whole-cell biocatalyst. Enzyme and Microbial Technology, 135, 109498.
Funding
This work was funded by “S&T Program of Hebei” (B2021208018).
Author information
Authors and Affiliations
Contributions
CS contributed to search the articles and write the paper. YW wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
1Recent discovery of natural and non-natural peroxidase
2Application of peroxidase in synthesis and degradation
3To provide reference of peroxidase participated in fine chemical industry
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, C., Wang, Y. Recent Progress on Peroxidase Modification and Application. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-023-04835-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04835-w