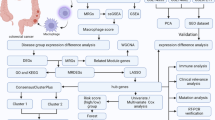
Abstract
Colon cancer (CC) is a primary human malignancy. Recently, the mechanism of the tumor microenvironment (TME) in CC has been a hot topic of research. However, there is uncertainty regarding the contribution of M2 macrophages and related genes to the prognosis for CC. M2 macrophage-related genes (M2RGs) were obtained from The Cancer Genome Atlas (TCGA) database. Immune cell infiltration in CC tissue was assessed by Cibersort. Based on the TCGA-COAD training set, a Least Absolute Shrinkage and Selection Operator (LASSO) Cox risk model was constructed and its efficiency was evaluated by analyzing risk profiles and survival profiles. Using gene set enrichment analysis (GSEA), the functional distinctions between high-risk and low-risk categories were further investigated. Finally, potential immune checkpoints, immunotherapy efficiency, and clinical treatment of high-risk patients were evaluated. A total of 1063 M2RGs were identified in TCGA-COAD, 32 of these were confirmed to be strongly related to overall survival (OS), and 14 of these were picked to construct an OS-oriented prognostic model in CC patients. The M2RG signature had a positive correlation with unfavorable prognosis according to the survival analysis. Correlation analysis revealed that the risk model was positively associated with clinicopathological characteristics, immune cell infiltration, immune checkpoint inhibitor targets, the risk of immune escape, and the efficiency of anti-cancer medications. The risk model created using M2RGs may be useful in predicting the prognosis of CC.









Similar content being viewed by others
Data Availability
The TCGA, GEO, GEPIA and HPA databases are publicly available.
References
Bussard, K. M., Mutkus, L., Stumpf, K., Gomez-Manzano, C., & Marini, F. C. (2016). Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Research: BCR, 18(1), 84. https://doi.org/10.1186/s13058-016-0740-2
Matos, A. I., Carreira, B., Peres, C., Moura, L. I. F., Conniot, J., Fourniols, T., … Florindo, H. F. (2019). Nanotechnology is an important strategy for combinational innovative chemo-immunotherapies against colorectal cancer. Journal of Controlled Release: Official Journal of the Controlled Release Society, 307, 108–138. https://doi.org/10.1016/j.jconrel.2019.06.017
Gabrilovich, D. I., Ostrand-Rosenberg, S., & Bronte, V. (2012). Coordinated regulation of myeloid cells by tumours. Nature Reviews Immunology, 12(4), 253–268. https://doi.org/10.1038/nri3175
Fridman, W. H., Pagès, F., Sautès-Fridman, C., & Galon, J. (2012). The immune contexture in human tumours: Impact on clinical outcome. Nature Reviews Cancer, 12(4), 298–306. https://doi.org/10.1038/nrc3245
Wang, H., Tian, T., & Zhang, J. (2021). Tumor-associated macrophages (TAMs) in colorectal cancer (CRC): From mechanism to therapy and prognosis. International Journal of Molecular Sciences, 22(16), 8470. https://doi.org/10.3390/ijms22168470
Molgora, M., Esaulova, E., Vermi, W., Hou, J., Chen, Y., Luo, J., … Colonna, M. (2020). TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell, 182(4), 886–900.e17. https://doi.org/10.1016/j.cell.2020.07.013
Mosser, D. M., & Edwards, J. P. (2008). Exploring the full spectrum of macrophage activation. Nature Reviews. Immunology, 8(12), 958–969. https://doi.org/10.1038/nri2448
Guiducci, C., Vicari, A. P., Sangaletti, S., Trinchieri, G., & Colombo, M. P. (2005). Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Research, 65(8), 3437–3446. https://doi.org/10.1158/0008-5472.CAN-04-4262
Shabo, I., Olsson, H., Sun, X.-F., & Svanvik, J. (2009). Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. International Journal of Cancer, 125(8), 1826–1831. https://doi.org/10.1002/ijc.24506
Pinto, M. L., Rios, E., Durães, C., Ribeiro, R., Machado, J. C., Mantovani, A., … Oliveira, M. J. (2019). The two faces of tumor-associated macrophages and their clinical significance in colorectal cancer. Frontiers in Immunology, 10, 1875. https://doi.org/10.3389/fimmu.2019.01875
Waniczek, D., Lorenc, Z., Śnietura, M., Wesecki, M., Kopec, A., & Muc-Wierzgoń, M. (2017). Tumor-associated macrophages and regulatory T cells infiltration and the clinical outcome in colorectal cancer. Archivum Immunologiae Et Therapiae Experimentalis, 65(5), 445–454. https://doi.org/10.1007/s00005-017-0463-9
Shibutani, M., Maeda, K., Nagahara, H., Fukuoka, T., Nakao, S., Matsutani, S., … Ohira, M. (2017). The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: A retrospective study. BMC Cancer, 17(1), 404. https://doi.org/10.1186/s12885-017-3395-1
Väyrynen, J. P., Haruki, K., Lau, M. C., Väyrynen, S. A., Zhong, R., Dias Costa, A., … Nowak, J. A. (2021). The prognostic role of macrophage polarization in the colorectal cancer microenvironment. Cancer Immunology Research, 9(1), 8–19. https://doi.org/10.1158/2326-6066.CIR-20-0527
Algars, A., Irjala, H., Vaittinen, S., Huhtinen, H., Sundström, J., Salmi, M., … Jalkanen, S. (2012). Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. International Journal of Cancer, 131(4), 864–873. https://doi.org/10.1002/ijc.26457
Madden, K., & Kasler, M. K. (2019). Immune checkpoint inhibitors in lung cancer and melanoma. Seminars in Oncology Nursing, 35(5), 150932. https://doi.org/10.1016/j.soncn.2019.08.011
Smith, J. J., Deane, N. G., Wu, F., Merchant, N. B., Zhang, B., Jiang, A., … Beauchamp, R. D. (2010). Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology, 138(3), 958–968. https://doi.org/10.1053/j.gastro.2009.11.005
Mariathasan, S., Turley, S. J., Nickles, D., Castiglioni, A., Yuen, K., Wang, Y., … Powles, T. (2018). TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature, 554(7693), 544–548. https://doi.org/10.1038/nature25501
Wu, J., Li, L., Zhang, H., Zhao, Y., Zhang, H., Wu, S., & Xu, B. (2021). A risk model developed based on tumor microenvironment predicts overall survival and associates with tumor immunity of patients with lung adenocarcinoma. Oncogene, 40(26), 4413–4424. https://doi.org/10.1038/s41388-021-01853-y
Zeng, C., Liu, Y., He, R., Lu, X., Dai, Y., Qi, G., … Liu, Q. (2022). Identification and validation of a novel cellular senescence-related lncRNA prognostic signature for predicting immunotherapy response in stomach adenocarcinoma. Frontiers in Genetics, 13, 935056. https://doi.org/10.3389/fgene.2022.935056
Van der Jeught, K., Xu, H.-C., Li, Y.-J., Lu, X.-B., & Ji, G. (2018). Drug resistance and new therapies in colorectal cancer. World Journal of Gastroenterology, 24(34), 3834–3848. https://doi.org/10.3748/wjg.v24.i34.3834
Canton, J. (2014). Phagosome maturation in polarized macrophages. Journal of Leukocyte Biology, 96(5), 729–738. https://doi.org/10.1189/jlb.1MR0114-021R
Ladanyi, A., Mukherjee, A., Kenny, H. A., Johnson, A., Mitra, A. K., Sundaresan, S., … Lengyel, E. (2018). Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene, 37(17), 2285–2301. https://doi.org/10.1038/s41388-017-0093-z
Calvo, D., Gómez-Coronado, D., Suárez, Y., Lasunción, M. A., & Vega, M. A. (1998). Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. Journal of Lipid Research, 39(4), 777–788.
Montero-Calle, A., Gómez de Cedrón, M., Quijada-Freire, A., Solís-Fernández, G., López-Alonso, V., Espinosa-Salinas, I., … Barderas, R. (2022). Metabolic reprogramming helps to define different metastatic tropisms in colorectal cancer. Frontiers in Oncology, 12, 903033. https://doi.org/10.3389/fonc.2022.903033
Chen, C.-H., Ho, H.-H., Jiang, W.-C., Ao-Ieong, W.-S., Wang, J., Orekhov, A. N., … Yet, S.-F. (2022). Cysteine-rich protein 2 deficiency attenuates angiotensin II-induced abdominal aortic aneurysm formation in mice. Journal of Biomedical Science, 29(1), 25. https://doi.org/10.1186/s12929-022-00808-z
Novelli, D., Fumagalli, F., Staszewsky, L., Ristagno, G., Olivari, D., Masson, S., … Latini, R. (2019). Monocrotaline-induced pulmonary arterial hypertension: Time-course of injury and comparative evaluation of macitentan and Y-27632, a Rho kinase inhibitor. European Journal of Pharmacology, 865, 172777. https://doi.org/10.1016/j.ejphar.2019.172777
Abisambra, J., Jinwal, U. K., Miyata, Y., Rogers, J., Blair, L., Li, X., … Dickey, C. A. (2013). Allosteric heat shock protein 70 inhibitors rapidly rescue synaptic plasticity deficits by reducing aberrant tau. Biological Psychiatry, 74(5), 367–374. https://doi.org/10.1016/j.biopsych.2013.02.027
Field, C. J., Perez, A. M., Samet, T., Ricles, V., Iovine, M. K., & Lowe-Krentz, L. J. (2022). Involvement of transmembrane protein 184a during angiogenesis in zebrafish embryos. Frontiers in Physiology, 13, 845407. https://doi.org/10.3389/fphys.2022.845407
Sui, C., Wang, G., Chen, Q., & Ma, J. (2014). Variation risks of SFRP2 hypermethylation between precancerous disease and colorectal cancer. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine, 35(10), 10457–10465. https://doi.org/10.1007/s13277-014-2313-2
Li, D., Bi, F.-F., Chen, N.-N., Cao, J.-M., Sun, W.-P., Zhou, Y.-M., … Yang, Q. (2014). Epigenetic repression of phosphatidylethanolamine N-methyltransferase (PEMT) in BRCA1-mutated breast cancer. Oncotarget, 5(5), 1315–1325. https://doi.org/10.18632/oncotarget.1800
Umeda, S., Kanda, M., Shimizu, D., Nakamura, S., Sawaki, K., Inokawa, Y., … Kodera, Y. (2022). Lysosomal-associated membrane protein family member 5 promotes the metastatic potential of gastric cancer cells. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 25(3), 558–572. https://doi.org/10.1007/s10120-022-01284-y
Wang, J., Song, Z., Ren, L., Zhang, B., Zhang, Y., Yang, X., … Feng, C. (2022). Pan-cancer analysis supports MAPK12 as a potential prognostic and immunotherapeutic target in multiple tumor types, including in THCA. Oncology Letters, 24(6), 445. https://doi.org/10.3892/ol.2022.13565
Ruan, W., Yang, Y., Yu, Q., Huang, T., Wang, Y., Hua, L., … Pan, R. (2021). FUT11 is a target gene of HIF1α that promotes the progression of hepatocellular carcinoma. Cell Biology International, 45(11), 2275–2286. https://doi.org/10.1002/cbin.11675
Zhang, P., Tang, W., Jiang, Y., Lyu, F., Liu, Z., Xiao, Y., & Wang, D. (2022). Dry and wet experiments reveal the significant role of FUT11 in clear cell renal cell carcinoma. International Immunopharmacology, 113(Pt B), 109447. https://doi.org/10.1016/j.intimp.2022.109447
Sato, S., Nakamura, T., Katagiri, T., Tsuchikawa, T., Kushibiki, T., Hontani, K., … Hirano, S. (2017). Molecular targeting of cell-permeable peptide inhibits pancreatic ductal adenocarcinoma cell proliferation. Oncotarget, 8(69), 113662–113672. https://doi.org/10.18632/oncotarget.21939
Pei, D., Xu, C., Wang, D., Shi, X., Zhang, Y., Liu, Y., … Zhu, H. (2022). A novel prognostic signature associated with the tumor microenvironment in kidney renal clear cell carcinoma. Frontiers in Oncology, 12, 912155. https://doi.org/10.3389/fonc.2022.912155
Reijneveld, J. F., Ocampo, T. A., Shahine, A., Gully, B. S., Vantourout, P., Hayday, A. C., … Van Rhijn, I. (2020). Human γδ T cells recognize CD1b by two distinct mechanisms. Proceedings of the National Academy of Sciences of the United States of America, 117(37), 22944–22952. https://doi.org/10.1073/pnas.2010545117
Tang, M., Chen, J., Zeng, T., Ye, D.-M., Li, Y.-K., Zou, J., & Zhang, Y.-P. (2022). Systemic analysis of the DNA replication regulator origin recognition complex in lung adenocarcinomas identifies prognostic and expression significance. Cancer Medicine. https://doi.org/10.1002/cam4.5238
Baines, C. P., Kaiser, R. A., Sheiko, T., Craigen, W. J., & Molkentin, J. D. (2007). Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nature Cell Biology, 9(5), 550–555. https://doi.org/10.1038/ncb1575
The lncRNA BDNF-AS/WDR5/FBXW7 axis mediates ferroptosis in gastric cancer peritoneal metastasis by regulating VDAC3 ubiquitination - PubMed. (n.d.). Retrieved January 20, 2023, from https://pubmed.ncbi.nlm.nih.gov/35280682/
Edin, S., Wikberg, M. L., Dahlin, A. M., Rutegård, J., Öberg, Å., Oldenborg, P.-A., & Palmqvist, R. (2012). The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One, 7(10), e47045. https://doi.org/10.1371/journal.pone.0047045
Hu, Z., Teng, X.-L., Zhang, T., Yu, X., Ding, R., Yi, J., … Zou, Q. (2021). SENP3 senses oxidative stress to facilitate STING-dependent dendritic cell antitumor function. Molecular Cell, 81(5), 940–952.e5. https://doi.org/10.1016/j.molcel.2020.12.024
Ammendola, M., Sacco, R., Donato, G., Zuccalà, V., Russo, E., Luposella, M., … Ranieri, G. (2013). Mast cell positivity to tryptase correlates with metastatic lymph nodes in gastrointestinal cancer patients treated surgically. Oncology, 85(2), 111–116. https://doi.org/10.1159/000351145
Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer - PubMed. (n.d.). Retrieved February 5, 2023, from https://pubmed.ncbi.nlm.nih.gov/17645466/
Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas - PubMed. (n.d.). Retrieved February 5, 2023, from https://pubmed.ncbi.nlm.nih.gov/15888280/
Prognostic significance of eosinophils and mast cells in rectal cancer: Findings from the National Surgical Adjuvant Breast and Bowel Project (protocol R-01) - PubMed. (n.d.). Retrieved February 5, 2023, from https://pubmed.ncbi.nlm.nih.gov/2562788/
Mao, Y., Feng, Q., Zheng, P., Yang, L., Zhu, D., Chang, W., … Xu, J. (2018). Low tumor infiltrating mast cell density confers prognostic benefit and reflects immunoactivation in colorectal cancer. International Journal of Cancer, 143(9), 2271–2280. https://doi.org/10.1002/ijc.31613
A new dawn for eosinophils in the tumour microenvironment - PubMed. (n.d.). Retrieved February 6, 2023, from https://pubmed.ncbi.nlm.nih.gov/32678342/
Astigiano, S., Morandi, B., Costa, R., Mastracci, L., D’Agostino, A., Ratto, G. B., … Frumento, G. (2005). Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia (New York, N.Y.), 7(4), 390–396. https://doi.org/10.1593/neo.04658
Recurrent R-spondin fusions in colon cancer | Nature. (n.d.). Retrieved February 6, 2023, from https://jlu.doc110.com/https/6a6c7576706e6973746865676f6f642146ab1ccaa48adfb6349e6380fd54/articles/nature11282
Frenel, J.-S., Le Tourneau, C., O’Neil, B., Ott, P. A., Piha-Paul, S. A., Gomez-Roca, C., … Varga, A. (2017). Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: Results from the phase Ib KEYNOTE-028 trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 35(36), 4035–4041. https://doi.org/10.1200/JCO.2017.74.5471
Majidpoor, J., & Mortezaee, K. (2021). The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clinical Immunology (Orlando, Fla.), 226, 108707. https://doi.org/10.1016/j.clim.2021.108707
Zengin, M., Zergeroğlu, S., Okcu, O., & Benek, S. (2021). PD-1 and PD-L2 expression predict relapse risk and poor survival in patients with stage III colorectal cancer. Cellular Oncology (Dordrecht), 44(2), 423–432. https://doi.org/10.1007/s13402-020-00579-5
Huang, K.C.-Y., Chiang, S.-F., Chen, T.-W., Chen, W.T.-L., Yang, P.-C., Ke, T.-W., & Chao, K. S. C. (2020). Prognostic relevance of programmed cell death 1 ligand 2 (PDCD1LG2/PD-L2) in patients with advanced stage colon carcinoma treated with chemotherapy. Scientific Reports, 10(1), 22330. https://doi.org/10.1038/s41598-020-79419-3
PD-L2 expression in colorectal cancer: Independent prognostic effect and targetability by deglycosylation - PubMed. (n.d.). Retrieved February 6, 2023, from https://pubmed.ncbi.nlm.nih.gov/28811964/
Sridhar, S. S., & Goodwin, P. J. (2009). Insulin-insulin-like growth factor axis and colon cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 27(2), 165–167. https://doi.org/10.1200/JCO.2008.19.8937
Solomon, J. P., & Hechtman, J. F. (2019). Detection of NTRK fusions: Merits and limitations of current diagnostic platforms. Cancer Research, 79(13), 3163–3168. https://doi.org/10.1158/0008-5472.CAN-19-0372
Pietrantonio, F., Di Nicolantonio, F., Schrock, A. B., Lee, J., Tejpar, S., Sartore-Bianchi, A., … Cremolini, C. (2017). ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. Journal of the National Cancer Institute, 109(12). https://doi.org/10.1093/jnci/djx089
PI3K therapy reprograms mitochondrial trafficking to fuel tumor cell invasion - PubMed. (n.d.). Retrieved February 7, 2023, from https://pubmed.ncbi.nlm.nih.gov/26124089/.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MJ and YC; figure and table preparation was performed by LZ, LY, and CH. The first draft of the manuscript was written by MJ, and the draft of the manuscript was revised and supplemented by LL. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Ethical approval was not required.
Consent for Publication
All authors agreed to publish the research in this journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, M., Chen, Y., Zhang, L. et al. Construction and Evaluation of an M2 Macrophage-Related Prognostic Model for Colon Cancer. Appl Biochem Biotechnol (2023). https://doi.org/10.1007/s12010-023-04789-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04789-z