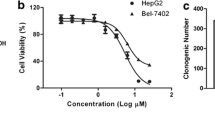
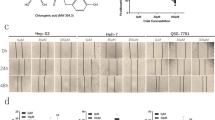
Abstract
Asperulosidic acid (ASPA) is a plant-extracted iridoid terpenoid with tumor-suppressive and anti-inflammatory properties. At present, the antitumor function of ASPA and its related mechanisms in hepatocellular carcinoma (HCC) cells were explored. Human normal hepatocytes HL-7702 and HCC cells (Huh7 and HCCLM3) were treated with varying concentrations (0 to 200 μg/mL) of ASPA. Cell viability, proliferation, apoptosis, migration, and invasion were checked. The expression of proteins was detected by Western blot. Furthermore, the effect of ASPA (100 μg/mL) on the sensitivity of HCC cells to chemotherapeutic agents, including doxorubicin and cisplatin, was evaluated. A subcutaneous xenografted tumor model was set up in nude mice, and the antitumor effects of ASPA were evaluated. ASPA hindered HCC cells’ proliferation, migration, and invasion, and amplified their apoptosis and sensitivity to chemotherapeutic agents. Additionally, ASPA inactivated the MEKK1/NF-κB pathway. Overexpression of MEKK1 increased HCC proliferation, migration, and invasion and facilitated chemoresistance. ASPA treatment alleviated the carcinogenic effect mediated by MEKK1 overexpression. MEKK1 knockdown slowed down HCC progression. However, ASPA could not exert additional antitumor effects in MEKK1 knockdown cells. In vivo results displayed that ASPA substantially curbed tumor growth and inactivated the MEKK1/NF-κB pathway in mice. All over, ASPA exerts antitumor effects in HCC by suppressing the MEKK1/NF-κB pathway.







Similar content being viewed by others

Data Availability
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA :
-
One-way analysis of variance
- ASPA :
-
Asperulosidic acid
- CCK8 :
-
Cell Counting Kits-8
- CDK2 :
-
Cyclin-dependent kinase 2
- CDDP :
-
Cisplatin;
- Dox :
-
Doxorubicin
- EMT :
-
Epithelial-mesenchymal transition
- HCC :
-
Hepatocellular carcinoma;
- IHC :
-
Immunohistochemistry
- ING4 :
-
Inhibitor of growth 4
- MEKK1 :
-
Mitogen-activated protein kinase kinase kinase 1
- NC :
-
Negative control
- NS :
-
No significance
- NF-κB :
-
Nuclear factor-kappaB
- OD :
-
Optical density
- PBS :
-
Phosphate-buffered solution
- RT-PCR :
-
Reverse transcription-polymerase chain reaction
- SD :
-
Standard deviation
- WB :
-
Western blot
References
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 71(3), 209–249.
Petrick, J. L., Florio, A. A., Znaor, A., Ruggieri, D., Laversanne, M., Alvarez, C. S., Ferlay, J., Valery, P. C., Bray, F., & McGlynn, K. A. (2020). International trends in hepatocellular carcinoma incidence, 1978–2012. International journal of cancer, 147(2), 317–330.
Yu, S. J. (2016). A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clinical and MolecularHepatology, 22(1), 7–17.
Grandhi, M. S., Kim, A. K., Ronnekleiv-Kelly, S. M., Kamel, I. R., Ghasebeh, M. A., & Pawlik, T. M. (2016). Hepatocellular carcinoma: From diagnosis to treatment. Surgical Oncology, 25(2), 74–85.
Zhang, W., Zhang, B., & Chen, X. P. (2021). Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Frontiers of Medicine, 15(2), 155–169.
Llovet, J. M., Montal, R., Sia, D., & Finn, R. S. (2018). Molecular therapies and precision medicine for hepatocellular carcinoma. Nature Reviews. Clinical Oncology, 15(10), 599–616.
Ye, J. H., Liu, M. H., Zhang, X. L., & He, J. Y. (2015). Chemical profiles and protective effect of Hedyotis diffusa Willd in lipopolysaccharide-induced renal inflammation mice. International Journal of Molecular Sciences, 16(11), 27252–27269.
Qian, K., Fu, D., Jiang, B., Wang, Y., Tian, F., Song, L., & Li, L. (2021). Mechanism of Hedyotis diffusa in the treatment of cervical cancer. Frontiers in Pharmacology, 12, 808144.
Chen, R., He, J., Tong, X., Tang, L., & Liu, M. (2016). The Hedyotis diffusa Willd. (Rubiaceae): A review on phytochemistry, pharmacology, quality control and pharmacokinetics. Molecules (Basel Switzerland), 21(6), 710.
Chen, X. Z., Cao, Z. Y., Chen, T. S., Zhang, Y. Q., Liu, Z. Z., Su, Y. T., Liao, L. M., & Du, J. (2012). Water extract of Hedyotis diffusa Willd suppresses proliferation of human HepG2 cells and potentiates the anticancer efficacy of low-dose 5-fluorouracil by inhibiting the CDK2-E2F1 pathway. Oncology Reports, 28(2), 742–748.
Zhao, C., Wei, M., Zheng, Y., Tao, W., Lv, Q., Wang, Q., Wang, S., & Chen, Y. (2021). The analyses of chemical components from Oldenlandia hedyotidea (DC.) Hand-Mazz and anticancer effects in vitro. Frontiers in Pharmacology, 12, 624296.
Filipčík, P., Latham, S. L., Cadell, A. L., Day, C. L., Croucher, D. R., & Mace, P. D. (2020). A cryptic tubulin-binding domain links MEKK1 to curved tubulin protomers. Proceedings of the National Academy of Sciences of the United States of America, 117(35), 21308–21318.
Yan, M., Dai, T., Deak, J. C., Kyriakis, J. M., Zon, L. I., Woodgett, J. R., & Templeton, D. J. (1994). Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature, 372(6508), 798–800.
Lee, F. S., Peters, R. T., Dang, L. C., & Maniatis, T. (1998). MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta. Proceedings of the National Academy of Sciences of the United States of America, 95(16), 9319–9324.
Liu, C., Wang, S., Zhu, S., Wang, H., Gu, J., Gui, Z., Jing, J., Hou, X., & Shao, Y. (2016). MAP3K1-targeting therapeutic artificial miRNA suppresses the growth and invasion of breast cancer in vivo and in vitro. Springerplus, 5, 11.
Su, F., Li, H., Yan, C., Jia, B., Zhang, Y., & Chen, X. (2009). Depleting MEKK1 expression inhibits the ability of invasion and migration of human pancreatic cancer cells. Journal of cancer research and clinical oncology, 135(12), 1655–1663.
Chauhan, A., Islam, A. U., Prakash, H., & Singh, S. (2022). Phytochemicals targeting NF-κB signaling: Potential anti-cancer interventions. Journal of pharmaceutical analysis, 12(3), 394–405.
Begum, S. M. F. M., & Hemalatha, S. (2022). Gelidiella acerosa compounds target NFκB cascade in lung adenocarcinoma. Applied biochemistry and biotechnology, 194(4), 1566–1579.
Ha, S. E., Kim, S. M., Vetrivel, P., Kim, H. H., Bhosale, P. B., Heo, J. D., Lee, H. J., & Kim, G. S. (2021). Inhibition of cell proliferation and metastasis by scutellarein regulating PI3K/Akt/NF-κB signaling through PTEN activation in hepatocellular carcinoma. International journal of molecular sciences, 22(16), 8841.
Habiba, Y. H., Omran, G. A., Helmy, M. W., & Houssen, M. E. (2022). Antitumor effects of rhamnazinon sorafenib-treated human hepatocellular carcinoma cell lines via modulation of VEGF signaling and PI3K/NF-κB p38/caspase-3 axes cross talk. Life sciences, 297, 120443.
Wu, Q., Gai, S., & Zhang, H. (2022). Asperulosidic acid, a bioactive iridoid, alleviates placental oxidative stress and inflammatory responses in gestational diabetes mellitus by suppressing NF-κB and MAPK signaling pathways. Pharmacology, 107(3–4), 197–205.
Akateh, C., Black, S. M., Conteh, L., Miller, E. D., Noonan, A., Elliott, E., Pawlik, T. M., Tsung, A., & Cloyd, J. M. (2019). Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World journal of gastroenterology, 25(28), 3704–3721.
Liu, X., Li, M., Wang, X., Dang, Z., Yu, L., Wang, X., Jiang, Y., & Yang, Z. (2019). Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepatocellular carcinoma. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 62, 152930.
Xiang, Y., Guo, Z., Zhu, P., Chen, J., & Huang, Y. (2019). Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Medicine, 8(5), 1958–1975.
Qi, F., Li, A., Inagaki, Y., Gao, J., Li, J., Kokudo, N., Li, X. K., & Tang, W. (2010). Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Bioscience Trends, 4(6), 297–307.
Hou, J., Shi, K., Liu, Y., Chen, J., Ran, C., & Wang, X. (2022). Effects of traditional Chinese medicine adjuvant therapy on the survival of patients with primary liver cancer. Evidence-based Complementary and Alternative Medicine : ECAM, 2022, 9810036.
Shen, Z., Cheng, H., Shen, W., Tao, L., Zeng, Y., Wu, M., Sun, D., & GuWenzhe, D. O. O. (2017). Effect of Aidi injection plus transarterial chemoembolization on primary hepatic carcinoma: A systematic review and Meta-analysis. Journal of Traditional Chinese Medicine, 37(5), 567–587.
Chen, S. L., Ho, C. Y., Lin, W. C., Lee, C. W., Chen, Y. C., Chen, J. L., & Chen, H. Y. (2022). The characteristics and mortality of Chinese herbal medicine users among newly diagnosed inoperable huge hepatocellular carcinoma (≥10 cm) patients: A retrospective cohort study with exploration of core herbs. International Journal of Environmental Research and Public Health, 19(19), 12480.
Wang, C., Xin, P., Wang, Y., Zhou, X., Wei, D., Deng, C., & Sun, S. (2018). Iridoids and sfingolipids from Hedyotis diffusa. Fitoterapia, 124, 152–159.
Lu, Y., Guan, T., Xu, S., Chen, Y. E., Shen, Q., Zhu, S., Liu, Y., Liang, J., & Hou, S. (2022). Asperuloside inhibited epithelial-mesenchymal transition in colitis associated cancer via activation of vitamin D receptor. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 101, 154070.
Bai, G., Chen, B., Xiao, X., Li, Y., Liu, X., Zhao, D., Zhang, L., & Zhao, D. (2022). Geniposide inhibits cell proliferation and migration in human oral squamous carcinoma cells via AMPK and JNK signaling pathways. Experimental and Therapeutic Medicine, 24(6), 706.
Li, L., & Wang, H. (2016). Heterogeneity of liver cancer and personalized therapy. Cancer Letters, 379(2), 191–197.
Schichl, Y. M., Resch, U., Lemberger, C. E., Stichlberger, D., & de Martin, R. (2011). Novel phosphorylation-dependent ubiquitination of tristetraprolin by mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (MEKK1) and tumor necrosis factor receptor-associated factor 2 (TRAF2). The Journal of Biological Chemistry, 286(44), 38466–38477.
Witowsky, J. A., & Johnson, G. L. (2003). Ubiquitylation of MEKK1 inhibits its phosphorylation of MKK1 and MKK4 and activation of the ERK1/2 and JNK pathways. The Journal of Biological Chemistry, 278(3), 1403–1406.
Komoda, F., Shino, Y., Hirano, T., Okutomi, Y., Okamoto, H., Hayashi, Y., Suyama, T., Ebara, M., Saisho, H., & Shirasawa, H. (2002). MEKK1 induces c-Jun complexes that act as negative regulators for cell survival and proliferation of HCC cells. International Journal of Oncology, 21(3), 553–559.
Gentile, S., Eskandari, N., Rieger, M. A., & Cuevas, B. D. (2021). MEKK1 regulates chemokine expression in mammary fibroblasts: Implications for the breast tumor microenvironment. Frontiers in Oncology, 11, 609918.
Xia, Y., Yang, W., Bu, W., Ji, H., Zhao, X., Zheng, Y., Lin, X., Li, Y., & Lu, Z. (2013). Differential regulation of c-Jun protein plays an instrumental role in chemoresistance of cancer cells. The Journal of Biological Chemistry, 288(27), 19321–19329.
Kan, Z., Jaiswal, B. S., Stinson, J., Janakiraman, V., Bhatt, D., Stern, H. M., Yue, P., Haverty, P. M., Bourgon, R., Zheng, J., Moorhead, M., Chaudhuri, S., Tomsho, L. P., Peters, B. A., Pujara, K., Cordes, S., Davis, D. P., Carlton, V. E., Yuan, W., Li, L., … Seshagiri, S. (2010). Diverse somatic mutation patterns and pathway alterations in human cancers. Nature, 466(7308), 869–873.
Sauvant, C., Nowak, M., Wirth, C., Schneider, B., Riemann, A., Gekle, M., & Thews, O. (2008). Acidosis induces multi-drug resistance in rat prostate cancer cells (AT1) in vitro and in vivo by increasing the activity of the p-glycoprotein via activation of p38. International Journal of Cancer, 123(11), 2532–2542.
Zhao, L., Wang, Y., Jiang, L., He, M., Bai, X., Yu, L., & Wei, M. (2016). MiR-302a/b/c/d cooperatively sensitizes breast cancer cells to adriamycin via suppressing P-glycoprotein(P-gp) by targeting MAP/ERK kinase kinase 1 (MEKK1). Journal of Experimental & Clinical Cancer Research : CR, 35, 25.
Zhang, G., He, J., Ye, X., Zhu, J., Hu, X., Shen, M., Ma, Y., Mao, Z., Song, H., & Chen, F. (2019). β-Thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ROS-mediated Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma. Cell Death & Disease, 10(4), 255.
Rasmi, R. R., Sakthivel, K. M., & Guruvayoorappan, C. (2020). NF-κB inhibitors in treatment and prevention of lung cancer. Biomedicine & Pharmacotherapy Biomedecine & Pharmacotherapie, 130, 110569.
Hoesel, B., & Schmid, J. A. (2013). The complexity of NF-κB signaling in inflammation and cancer. Molecular Cancer, 12, 86.
Nakano, H., Shindo, M., Sakon, S., Nishinaka, S., Mihara, M., Yagita, H., & Okumura, K. (1998). Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proceedings of the National Academy of Sciences of the United States of America, 95(7), 3537–3542.
He, G., & Karin, M. (2011). NF-κB and STAT3 - Key players in liver inflammation and cancer. Cell Research, 21(1), 159–168.
Jiang, X. J., Chen, Z. W., Zhao, J. F., Liao, C. X., Cai, Q. H., & Lin, J. (2021). cIAP2 via NF-κB signalling affects cell proliferation and invasion in hepatocellular carcinoma. Life Sciences, 266, 118867.
Qian, F., Hu, Q., Tian, Y., Wu, J., Li, D., Tao, M., Qin, L., Shen, B., & Xie, Y. (2019). ING4 suppresses hepatocellular carcinoma via a NF-κB/miR-155/FOXO3a signaling axis. International Journal of Biological Sciences, 15(2), 369–385.
Funding
The work was funded by grants from the Key Discipline of Hepatobiliary and Pancreatic Surgery of Jiaxing City [2023-ZC-005], Translational therapy center for hepatobiliary pancreatic cancer [2021-YJZX-04].
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Huiwen Qiu; performed the experiments: Liang Li; statistical analysis: Liang Li; wrote the paper: Liang Li, Huiwen Qiu. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Our study was approved by the Ethics Committee of The First Hospital of Jiaxing.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Qiu, H. Asperulosidic Acid Restrains Hepatocellular Carcinoma Development and Enhances Chemosensitivity Through Inactivating the MEKK1/NF-κB Pathway. Appl Biochem Biotechnol 196, 1–17 (2024). https://doi.org/10.1007/s12010-023-04500-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04500-2