Abstract
Plants, rich in phytocompounds, have been in usage since time immemorial for treating various diseases, namely, cancer. One such plant species, Allium ascalonicum (Shallot) belonging to Amaryllidaceae family is being studied here for its anti-carcinogenic properties against breast cancer. GC–MS characterization of A. ascalonicum exhibited 48 phytocompounds containing five peak phytocompounds and 13 phytocompounds with anti-carcinogenic properties. These 13 anti-carcinogenic phytocompounds were docked with three hormonal receptors involved in breast cancer malignancy, namely, ERα, PR, and human EGFR with tamoxifen as standard for in silico analysis. The results exhibited three phytocompounds that had better binding scores compared to that of the standard drug, tamoxifen. Lyophilized powder of aqueous A. ascalonicum extract, also referred as ASE, was used for in vitro approaches. Antioxidant study using DPPH assay revealed that the highest percentage of FRSA in ASE, nearly 51%, was observed at 50 µg/ml concentration. Cytotoxicity study on MCF-7 cell line using MTT assay demonstrated IC50 value at 1400 µg/ml and anti-proliferative study using Trypan blue assay for the determination of percentage viability of MCF-7 cells at IC50 concentration was observed to be 49%. Anti-mitotic activity using Vigna radiata seed germination assay revealed clear morphological differences in a dose-dependent manner between the seeds grown at various concentrations of ASE with nearly 56.5% growth inhibition observed at 1500 µg/ml concentration. Hence, this research work proves that Allium ascalonicum has very good anti-carcinogenic properties and this can be confirmed further through in vivo animal model studies and it can also be formulated as a promising drug to treat breast cancer.
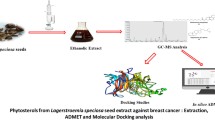
Graphical Abstract
GC–MS characterization of Allium ascalonicum demonstrated the presence of five peak compounds and thirteen anti-carcinogenic compounds. The thirteen anti-carcinogenic compounds were docked with three target proteins (in silico analysis) involved in breast cancer malignancy and identified the presence of three potential phytocompounds that can be used for treating breast cancer. In vitro approaches also confirmed the presence of anti-carcinogenic properties such as antioxidative potential, cytotoxic, anti-proliferative, and anti-mitotic effects. Hence, Allium ascalonicum can be taken further to in vivo studies so that it can be formulated to treat breast cancer.











Similar content being viewed by others
Data Availability
Data will be available on request
Code Availability
Not applicable
Abbreviations
- GC-MS:
-
Gas chromatography-mass spectrometry
- A. ascalonicum :
-
Allium ascalonicum
- ERα:
-
Alpha binding domain-human estrogen receptor
- PR:
-
Human progesterone receptor
- EGFR:
-
Epidermal growth factor receptor
- ASE:
-
Aqueous shallot extract
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- FRSA:
-
Free radical scavenging activity
- MTT:
-
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- V. rad iata :
-
Vigna radiata
References
Iqbal, J., Abbasi, B. A., Batool, R., Mahmood, T., Ali, B., Khalil, A. T., Kanwal, S., Shah, S. A., & Ahmed, R. (2018). Potential phytocompounds for developing breast cancer therapeutics: Nature’s healing touch. European Journal of Pharmacology, 827, 125–148. https://doi.org/10.1016/j.ejphar.2018.03.007
Seigel, R. L., Miller, K. D., & Fuchs, H. E. J. A. (2022). Cancer statistics, 2022. CA: A Cancer Journal for Clinicians. https://doi.org/10.3322/caac.21708
Lei, S., Zheng, R., Zhang, S., Wang, S., Chen, R., Sun, K., Zeng, H., Zhou, J., & Wei, W. (2021). Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Communications, 41(11), 1183–1194. https://doi.org/10.1002/cac2.12207
Abinaya, M., Priya, S., Karunya, J. R., Ranjani, S., & Hemalatha, S. (2021). Screening the efficacy of compounds from ghee to control cancer: An in silico approach. Biointerface Research in Applied Chemistry, 11(6), 14115–14126. https://doi.org/10.33263/BRIAC116.1411514126
Asma, B., & Hemalatha, S. (2022). SARS-CoV-2 inhibitors from Nigella sativa. Applied Biochemistry and Biotechnology, 194, 1051–1090. https://doi.org/10.1007/s12010-021-03790-8
Asemani, Y., Zamani, N., Bayat, M., & Amirghofran, Z. (2019). Allium vegetables for possible future of cancer treatment. Phytotherapy Research, 33(12), 3019–3039. https://doi.org/10.1002/ptr.6490
Nicastro, H. L., Ross, S. A., & Milner, J. A. (2015). Garlic and onions: Their cancer prevention properties. Cancer Prevention Research, 8(3), 181–189. https://doi.org/10.1158/1940-6207.CAPR-14-0172
Yang, E. J., Kim, G. S., Kim, J. A., & Song, K. (2013). Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. PharmacognosyMagazine, 9(36), 302–308. https://doi.org/10.4103/0973-1296.117824
Kendler, B. (1987). Garlic (Allium sativum) and onion (Allium cepa): A review of their relationship to cardiovascular disease. Preventive Medicine, 16(5), 670–685. https://doi.org/10.1016/0091-7435(87)90050-8
Wang, Z. Y., & Yin, L. (2015). Estrogen receptor alpha-36 (ER-α36): A new player in human breast cancer. Molecular and Cellular Endocrinology, 418, 193–206. https://doi.org/10.1016/j.mce.2015.04.017
Kiani, J., Khan, A., Khawar, H., et al. (2006). Estrogen receptor α-negative and progesterone receptor-positive breast cancer: Lab error or real entity? Pathology and Oncology Research, 12, 223. https://doi.org/10.1007/BF02893416
Mueller, K. L., Yang, Z. Q., Haddad, R., Ethier, S. P., & Boerner, J. L. (2010). EGFR/Met association regulates EGFR TKI resistance in breast cancer. Journal of Molecular Signaling, 5, 1–8. https://doi.org/10.1186/1750-2187-5-8
Mir, M. A., Bashir, N., Alfaify, A., & Oteef, M. (2020). GC-MS analysis of Myrtus communis extract and its antibacterial activity against Gram-positive bacteria. BMC Complementary Medicine and Therapies, 20(1), 86. https://doi.org/10.1186/s12906-020-2863-3
Acharya, R., Chacko, S., Bose, P., Lapenna, A., & Pattanayak, S. (2019). Structure based multitargeted molecular docking analysis of selected furanocoumarins against breast cancer. Scientific Reports, 9(1), 15743. https://doi.org/10.1038/s41598-019-52162-0
Trott, O., & Olson, A. J. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455–461. https://doi.org/10.1002/jcc.21334
Ravindranath, K. J., Mohaideen, N. S. M. H., Srinivasan, H. (2022). Phytocompounds of onion target heat shock proteins (HSP70s) to control breast cancer malignancy. Applied Biochemistry and Biotechnology. Published online 6th June, 2022. https://doi.org/10.1007/s12010-022-04016-1
Blois, M. (1958). Antioxidant Determinations by the Use of a Stable Free Radical. Nature, 181, 1199–1200. https://doi.org/10.1038/1811199a0
Panneerselvam, C., Murugan, K., Roni, M., et al. (2016). Fern-synthesized nanoparticles in the fight against malaria: LC/MS analysis of Pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitology Research, 115, 997–1013. https://doi.org/10.1007/s00436-015-4828-x
Yen, G. C., & Duh, P. (1994). Scavenging effect of methanolic extracts of peanut hulls on free-radical and active oxygen species. Journal of Agricultural and Food Chemistry, 42(3), 629–632. https://doi.org/10.1021/jf00039a005
Al-Daghistani, H. I., Abu-Niaaj, L. F., Bustanji, Y., Al-Hamaideh, K. D., Al-Salamat, H., Nassar, M. N., Jaber, H. M., Amer, N. H., Abu-Irmaileh, B., & Al-Nuaimi, A. (2021). Antibacterial and cytotoxicity evaluation of Arum hygrophilum Bioss. European Review for Medical and Pharmacological Sciences, 25(23), 7306–7316. https://doi.org/10.26355/eurrev_202112_27424
Fredotović, Ž, Šprung, M., Soldo, B., et al. (2017). Chemical Composition and Biological Activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) Methanolic Extracts. Molecules, 22(3), 448. https://doi.org/10.3390/molecules22030448
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65(1–2), 55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Nigjeh, S. E., Yeap, S. K., Nordin, N., Kamalideghan, B., Ky, H., & Rosli, R. (2018). Citral induced apoptosis in MDA-MB-231 spheroid cells. BMC Complementary and Alternative Medicine, 18, 56. https://doi.org/10.1186/s12906-018-2115-y
Elsyana, V., Bintang, M., Priosoeryanto, B. (2016). Cytotoxicity and antiproliferative activity assay of clove mistletoe (Dendrophthoe pentandra (L.) Miq.) leaves extracts. Advances in Pharmacological and Pharmaceutical Sciences, 3242698. https://doi.org/10.1155/2016/3242698
Satyanarayana Murthy, G., Francis, T. P., Rajendra Singh, C., Nagendra, H. G., & Naik, C. (2011). An assay for screening anti-mitotic activity of herbal extracts. Current Science, 100, 1399–1404. https://www.currentscience.ac.in/Volumes/100/09/1399.pdf.
Osborne, C. (1998). Tamoxifen in the treatment of breast cancer. The New England Journal of Medicine, 339(22), 1609–1618. https://doi.org/10.1056/NEJM199811263392207
Qu, J., Zhao, X., Ma, P. X., & Guo, B. (2017). pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomaterialia, 58, 168–180. https://doi.org/10.1016/j.actbio.2017.06.001
Cecchi, L., Ieri, F., Vignolini, P., Mulinacci, N., & Romani, A. (2020). Characterization of volatile and flavonoid composition of different cuts of dried onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GCxGC-TOF and HPLC-DAD. Molecules, 25(2), 408. https://doi.org/10.3390/molecules25020408
Costa, E. V., Soares, L. D. N., Chaar, J. D. S., Silva, V. R., Santos, L. S., Koolen, H. H. F., Silva, F. M. A. D., Tavares, J. F., Zengin, G., Soares, M. B. P., & Bezerra, D. (2021). Benzylated dihydroflavones and isoquinoline-derived alkaloids from the Bark of Diclinanona calycina (Annonaceae) and their cytotoxicities. Molecules, 26(12), 3714. https://doi.org/10.3390/molecules26123714
Liguori, L., Califano, R., Albanese, D., Raimo, F., Crescitelli, A., Di Matteo, M. (2017). Chemical composition and antioxidant properties of five white onion (Allium cepa L.) landraces. Journal of Food Quality, 6873651. https://doi.org/10.1155/2017/6873651
Dalla Pozza, E., Dando, I., Pacchiana, R., Liboi, E., Scupoli, M. T., Donadelli, M., & Palmieri, M. (2020). Regulation of succinate dehydrogenase and role of succinate in cancer. Seminars in Cell and Developmental Biology, 98, 4–14. https://doi.org/10.1016/j.semcdb.2019.04.013
Vicker, N., Bailey, H. V., Day, J. M., Mahon, M. F., Smith, A., Tutill, H. J., Purohit, A., & Potter, B. (2021). Substituted aryl benzylamines as potent and selective inhibitors of 17β-hydroxysteroid dehydrogenase type 3. Molecules, 26(23), 7166. https://doi.org/10.3390/molecules26237166
Sumi, H., Yabuki, M., Iwai, K., Morimoto, M., Hibino, R., Inazuka, M., Hashimoto, K., Kosugi, Y., Aoyama, K., Yamamoto, S., Yoshimatsu, M., Yamasaki, H., Tozawa, R., Ishikawa, T., & Yoshida, S. (2013). Antitumor activity and pharmacodynamic biomarkers of a novel and orally available small-molecule antagonist of inhibitor of apoptosis proteins. Molecular Cancer Therapeutics, 12(2), 230–240. https://doi.org/10.1158/1535-7163.MCT-12-0699
Asano, M., Hashimoto, K., Saito, B., Shiokawa, Z., Sumi, H., Yabuki, M., Yoshimatsu, M., Aoyama, K., Hamada, T., Morishita, N., Dougan, D. R., Mol, C. D., Yoshida, S., & Ishikawa, T. (2013). Design, stereoselective synthesis, and biological evaluation of novel tri-cyclic compounds as inhibitor of apoptosis proteins (IAP) antagonists. Bioorganic and Medicinal Chemistry, 21(18), 5725–5737. https://doi.org/10.1016/j.bmc.2013.07.020
Jouffroy, M., Pye, P., Jerhaoui, S., Chen, W., & Surkyn, M. (2022). Development of a concise and robust route to a key fragment of MCL-1 inhibitors via stereoselective defluoroborylation. The Journal of Organic Chemistry, 87(4), 2136–2141. https://doi.org/10.1021/acs.joc.1c01666
Krishnamurth, Y. S., Yoda, H., Hiraoka, K., Inoue, T., Lin, J., Shinozaki, Y., Watanabe, T., Koshikawa, N., Takatori, A., & Nagase, H. (2021). Targeting the mutant PIK3CA gene by DNA-alkylating pyrrole-imidazole polyamide in cervical cancer. Cancer Science, 112(3), 1141–1149. https://doi.org/10.1111/cas.14785
Maeda, R., Bando, T., & Sugiyama, H. (2021). Application of DNA-alkylating pyrrole-imidazole polyamides for cancer treatment. ChemBioChem, 22(9), 1538–1545. https://doi.org/10.1002/cbic.202000752
Han, M., Shen, J., Wang, L., Wang, Y., Zhai, X., Li, Y., Liu, M., Li, Z., Zuo, D., & Wu, Y. (2018). 5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(2-methoxy-4-(4-((4-methylpiperazin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)phenyl)pyrimidine-2,4-diamine (WY-135), a novel ALK inhibitor, induces cell cycle arrest and apoptosis through inhibiting ALK and its downs. Chemico-Biological Interactions, 284, 24–31. https://doi.org/10.1016/j.cbi.2018.02.018
Wang, L., Xu, X., Liu, T., Wang, J., Shen, J., Guo, M., Wu, Y., Zhai, X., & Zuo, D. (2020). 1-(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)-3-methoxyphenyl)-3-(2-(dimethylamino)ethyl)imidazolidin-2-one (ZX-42), a novel ALK inhibitor, induces apoptosis and protective autophagy in H2228 cells. Journal of Pharmacy and Pharmacology, 72(10), 1370–1382. https://doi.org/10.1111/jphp.13315
Villière, A., Le Roy, S., Fillonneau, C., et al. (2015). Evaluation of aroma profile differences between sué, sautéed, and pan-fried onions using an innovative olfactometric approach. Flavour, 4, 24. https://doi.org/10.1186/s13411-015-0034-0
Kurutas, E. (2016). The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutrition Journal, 15, 71. https://doi.org/10.1186/s12937-016-0186-5
Etaiw, S. E., Abd El-Aziz, D. M., Abd El-Zaher, E. H., & Ali, E. (2011). Synthesis, spectral, antimicrobial and antitumor assessment of Schiff base derived from 2-aminobenzothiazole and its transition metal complexes. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 79(5), 1331–1337. https://doi.org/10.1016/j.saa.2011.04.064
Chia, PW., Lim, BS., Tan, KC., Yong, FS., Su-Yin, K. (2018). Water extract of onion peel for the synthesis of bisindolylmethanes. Journal of King Saud University - Science, 31(4).https://doi.org/10.1016/j.jksus.2018.05.029
Boot, A., Brito, A., Van Wezel, T., Morreau, H., Costa, M., & Proença, F. (2014). Anticancer activity of novel pyrido[2,3-b]indolizine derivatives: The relevance of phenolic substituents. Anticancer Research, 34(4), 1673–1677. https://ar.iiarjournals.org/content/34/4/1673.long.
Wermes, C., Cannon, R., Haasnoot, S., Colstee, H., Niedeveld, C., Koopmanschap, G., & Da Costa, N. (2017). Identification of thiols in yellow onion (Allium cepa L.) using solvent vented large volume injection GC-MS. Journal of Food Science, 82(11), 2606–2613. https://doi.org/10.1111/1750-3841.13943
LaVoie, E. J., Dolan, S., Little, P., Wang, C. X., Sugie, S., & Rivenson, A. (1988). Carcinogenicity of quinoline, 4- and 8-methylquinoline and benzoquinolines in newborn mice and rats. Food and Chemical Toxicology, 26(7), 625–629. https://doi.org/10.1016/0278-6915(88)90233-5
Fei, X., Gu, Y., Ban, Y., Liu, Z., & Zhang, B. (2009). Thiazole Orange derivatives: Synthesis, fluorescence properties, and labeling cancer cells. Bioorganic and Medicinal Chemistry, 17(2), 585–591. https://doi.org/10.1016/j.bmc.2008.11.083
Acknowledgements
The authors are very much grateful to the School of Life Sciences, B. S. Abdur Rahman Crescent Institute of Science and Technology, Chennai, for providing research facilities and also for their constant support and encouragement.
Funding
This project work was sanctioned by Tamil Nadu State Council for Science and Technology (TNSCST) under the Student Project Scheme (SPS)-Science Stream (2021–2022) Code: MS-017, DOTE Campus, Chennai-600 025, Tamil Nadu, India.
Author information
Authors and Affiliations
Contributions
Hemalatha Srinivasan: conceived the idea, supervision, data curation, validation, and project administration. Karunya Jenin Ravindranath: designed-performed research and wrote manuscript. Simon Durairaj Christian: performed research, analyzed data, reviewing, and editing.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
All authors contributed equally and approved the manuscript for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ravindranath, K.J., Christian, S.D. & Srinivasan, H. Screening of Anti-carcinogenic Properties of Phytocompounds from Allium ascalonicum for Treating Breast Cancer Through In Silico and In Vitro Approaches. Appl Biochem Biotechnol 195, 1136–1157 (2023). https://doi.org/10.1007/s12010-022-04202-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04202-1