Abstract
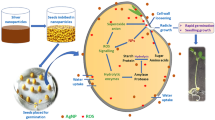
Biosynthesis of carnosic acid (CA), one of the most industrially valuable medicinal compounds present in rosemary (Rosmarinus officinalis L.) leaves, is affected by various plant stressors. In this study, effects of silver nanoparticle (AgNP) treatment on the secondary metabolism and CA production of rosemary plants were investigated. AgNP of 0, 25, 50, 100, and 200 ppm were utilized on hydroponically grown plants using foliar spray. Efficient absorbance and translocation of AgNPs to the plant roots were confirmed by XRF (X-ray fluorescence) analysis. The fluctuations of important antioxidant compounds such as CA content, phenolics, flavonoids, and acid ascorbic were analyzed and their correlations evaluated. Results revealed that application of 200 ppm AgNPs for 12 days increased CA level more than 11%, as compared to the control plants. Furthermore, significant positive correlations were observed between total flavonoids and CA content under AgNP treatment, suggesting that AgNP acted as an elicitor and triggered the enhancement of CA accumulation effectively. These data suggest that concentration-dependent AgNP may be used to boost antioxidant activity and phytochemical contents of other medicinal plants.







Similar content being viewed by others
Abbreviations
- AgNPs:
-
Silver nanoparticles
- DPPH:
-
1, 1-diphenyl-2-picrylhydrazyl
- TPTZ:
-
2, 4, 6-tripyridyl-s-triazine
- IC50 :
-
Concentration of extracts that reduce 50% of DPPH radicals
- AEAC:
-
Ascorbic acid equivalent antioxidant activity
- FRAP:
-
Ferric reducing antioxidant power
- GAE:
-
gallic acid equivalent
- AA:
-
Ascorbic acid
References
Luis, J. C., Martín Pérez, R., & Valdés González, F. (2007). UV-B radiation effects on foliar concentrations of rosmarinic and carnosic acids in rosemary plants. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 101, 1211–1215.
Tounekti, T., Munné-Bosch, S., Vadel, A. M., Chtara, C., & Khemira, H. (2010). Influence of ionic interactions on essential oil and phenolic diterpene composition of Dalmatian sage (Salvia officinalis L.). Plant Physiology and Biochemistry, 48, 813–821.
Tounekti, T., Vadel, A. M., Ennajeh, M., Khemira, H., & Munné-Bosch, S. (2011a). Ionic interactions and salinity affect monoterpene and phenolic diterpene composition in rosemary (Rosmarinus officinalis). Journal of Plant Nutrition and Soil Science, 174, 504–514.
Tounekti, T., Hernández, I., Muller, M., Khemira, H., & Munné-Bosch, S. (2011b). Kinetin applications alleviate salt stress and improve the antioxidant composition of leaf extracts in Salvia officinalis. Plant Physiology and Biochemistry, 49, 1165–1176.
Birtić, S., Dussort, P., Pierre, F.-X., Bily, A. C., & Roller, M. (2015). Carnosic acid. Phytochemistry, 115, 9–19.
Giraldo, J. P., Landry, M. P., Faltermeier, S. M., McNicholas, T. P., Iverson, N. M., Boghossian, A. A., Reuel, N. F., Hilmer, A. J., Sen, F., & Brew, J. A. (2014). Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nature Materials, 13, 400.
Sharma, P., Bhatt, D., Zaidi, M. G. H., Sardahi, P., Khana, P. K., & Arora, S. (2012). Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Applied Biochemistry and Biotechnology, 167(8), 2225–2233.
Azeez, L., Lateef, A., & Adebisi, S. A. (2017). Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Applied Nanoscience, 7, 59–66.
Krishnaraj, C., Jagan, E., Ramachandran, R., Abirami, S., Mohan, N., & Kalaichelvan, P. (2012). Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochemistry, 47, 651–658.
Salama, H. M. (2012). Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). International Research Journal of Biotechnology, 3, 190–197.
Qian, H., Peng, X., Han, X., Ren, J., Sun, L., & Fu, Z. (2013). Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. Journal of Environmental Sciences, 25, 1947–1956.
Amooaghaie, R., Saeri, M. R., & Azizi, M. (2015). Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotoxicology and Environmental Safety, 120, 400–408.
Gorczyca, A., Pociecha, E., Kasprowicz, M., & Niemiec, M. (2015). Effect of nanosilver in wheat seedlings and Fusarium culmorum culture systems. European Journal of Plant Pathology, 142, 251–261.
Galbraith, D. W. (2007). Nanobiotechnology: silica breaks through in plants. Nature Nanotechnology, 2, 272.
Torney, F., Trewyn, B. G., Lin, V. S., & Wang, K. (2007). Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nature Nanotechnology, 2(5), 295–300.
Eichert, T., Kurtz, A., Steiner, U., & Goldbach, H. E. (2008). Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiologia Plantarum, 134(1), 151–160.
Dhoke, S. K., Mahajan, P., Kamble, R., & Khanna, A. (2013). Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotechnology Development, 3.
Alidoust, D., & Isoda, A. (2013). Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): foliar spray versus soil amendment. Acta Physiologiae Plantarum, 35, 3365–3375.
Elzey, S., & Grassian, V. H. (2010). Agglomeration, isolation and dissolution of commercially manufactured silver nanoparticles in aqueous environments. Journal of Nanoparticle Research, 12, 1945–1958.
Asher, C., & Edwards, D. (1983). In Modern solution culture techniques. Inorganic plant nutrition. Springer, pp. 94–119.
Marguí, E., Queralt, I., & Hidalgo, M. (2009). Application of X-ray fluorescence spectrometry to determination and quantitation of metals in vegetal material. TrAC Trends in Analytical Chemistry, 28, 362–372.
Okamura, N., Fujimoto, Y., Kuwabara, S., & Yagi, A. (1994). High-performance liquid chromatographic determination of carnosic acid and carnosol in Rosmarinus officinalis L. and Salvia officinalis L. Journal of Chromatography A, 679, 381–386.
Cuendet, M., Hostettmann, K., & Potterat, O. (1997). Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helvetica Chimica Acta, 80, 1144–1152.
Meda, A., Lamien, C. E., Romito, M., Millogo, J., & Nacoulma, O. G. (2005). Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry, 91, 571–577.
Chang, S. S., Ostric-Matijasevic, B., Hsieh, O. A. L., & Huang, C.-L. (1977). Natural antioxidants from rosemary and sage. Journal of Food Science, 42, 1102–1106.
De Pinto, M., Francis, D., & De Gara, L. (1999). The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma, 209(1-2), 90–97.
Zheng, W., & Wang, S. Y. (2001). Antioxidant activity and phenolic compounds in selected herbs. Journal of Agricultural and Food Chemistry, 49(11), 5165–5170.
Chandra, S., Khan, S., Avula, B., Lata, H., Yang, M. H., ElSohly, M. A., & Khan, I. A. (2014). Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evidence-based Complementary and Alternative Medicine, 2014, 9.
Leonov, A., Arlia-Ciommo, A., Piano, A., Svistkova, V., Lutchman, V., Medkour, Y., & Titorenko, V. (2015). Longevity extension by phytochemicals. Molecules, 20(4), 6544–6572.
Khodakovskaya, M. V., Kim, B.-S., Kim, J. N., Alimohammadi, M., Dervishi, E., Mustafa, T., & Cernigla, C. E. (2013). Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small, 9, 115–123.
Raliya, R., Nair, R., Chavalmane, S., Wang, W.-N., & Biswas, P. (2015). Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics: Integrated Biometal Science, 7, 1584–1594.
Vats, S. (2016). Effect of initial temperature treatment on phytochemicals and antioxidant activity of Azadirachta indica A. Juss. Applied Biochemistry and Biotechnology, 178(3), 504–512.
Sharma, P., Jha, A. B., Dubey, R. S., & Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012.
Kole, C., Kole, P., Randunu, K. M., Choudhary, P., Podila, R., Ke, P. C., Rao, A. M., & Marcus, R. K. (2013). Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnology, 13, 37.
Munné-Bosch, S., & Alegre, L. (2003). Drought-induced changes in the redox state of alpha-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiology, 131, 1816–1825.
Medhe, S., Bansal, P., & Srivastava, M. M. (2014). Enhanced antioxidant activity of gold nanoparticle embedded 3,6-dihydroxyflavone: a combinational study. Applied Nanoscience, 4, 153–161.
Edreva, A., Velikova, V., Tsonev, T., Dagnon, S., Gürel, A., Aktaş, L., & Gesheva, E. (2008). Stress-protective role of secondary metabolites: diversity of functions and mechanisms. General and Applied Plant Physiology, 34, 67–78.
Minibayeva, F., Kolesnikov, O., Chasov, A., Beckett, R., Lüthje, S., Vylegzhanina, N., Buck, F., & Böttger, M. (2009). Wound-induced apoplastic peroxidase activities: their roles in the production and detoxification of reactive oxygen species. Plant, Cell & Environment, 32, 497–508.
Elstner, E., Wagner, G., & Schutz, W. (1988). Activated oxygen in green plants in relation to stress situations. Current topics in plant biochemistry and physiology: Proceedings of the Plant Biochemistry and Physiology Symposium held at the University of Missouri, Columbia (USA).
Witzell, J., Gref, R., & Näsholm, T. (2003). Plant-part specific and temporal variation in phenolic compounds of boreal bilberry (Vaccinium myrtillus) plants. Biochemical Systematics and Ecology, 31, 115–127.
Ahn, M.-R., Kumazawa, S., Usui, Y., Nakamura, J., Matsuka, M., Zhu, F., & Nakayama, T. (2007). Antioxidant activity and constituents of propolis collected in various areas of China. Food Chemistry, 101, 1383–1392.
Triantaphylides, C., Krischke, M., Hoeberichts, F. A., Ksas, B., Gresser, G., Havaux, M., Van Breusegem, F., & Mueller, M. J. (2008). Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiology, 148(2), 960–968.
Barbasz, A., Ocwieja, M., & Barbasz, J. (2015). Cytotoxic activity of highly purified silver nanoparticles sol against cells of human immune system. Applied Biochemistry and Biotechnology, 176(3), 817–834.
Benzie, I., & Strain, J. (1999). Ferric reducing (antioxidant) power as a measure of antioxidant capacity: the FRAP assay. Methods in Enzymology, 299, 15–27.
Acknowledgments
The authors are grateful to Prof. M. Shahbazi, the CEO of AryaTinaGene (ATG) Biopharmaceutical Company, Iran, for providing access to HPLC facilities; to Dr. A. Dehno Khalaji, Golestan University, for providing AgNPs; to M. R. Azarakhsh, for helping in drawing the graphs; and G. Bagheriehnajar for carefully reading the manuscript and improving the English. This research was supported by Golestan University in the form of a grant (41.5745) awarded to MHS and MBBN.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hadi Soltanabad, M., Bagherieh-Najjar, M.B. & Mianabadi, M. Carnosic Acid Content Increased by Silver Nanoparticle Treatment in Rosemary (Rosmarinus officinalis L.). Appl Biochem Biotechnol 191, 482–495 (2020). https://doi.org/10.1007/s12010-019-03193-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03193-w