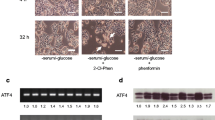
Abstract
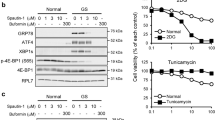
We recently characterized the cytotoxic action of a novel phenformin derivative, 2-(2-chlorophenyl)ethylbiguanide (2-Cl-Phen), on HT-29 cells under a serum- and glucose-deprived condition and found that 2-Cl-Phen attenuated ATF4 and GRP78, typical downstream targets of the unfolded protein response (UPR), together with c-Myc protein expression in a transcriptional and posttranscriptional manner. In the current study, we focused on the expression of ER-associated protein degradation (ERAD) components after treatment with 2-Cl-Phen under a serum- and glucose-deprived condition. Among nine ER-localizing factors regulating protein quality control within the ER, the amounts of Herp, GRP78, GRP94, and OS9 proteins were significantly downregulated by treatment with 2-Cl-Phen. In particular, replacement of the culture medium with the serum- and glucose-deprived medium induced the expression of Herp protein at the early phase. This increase in Herp protein was accompanied by an increase in its mRNA, and its induction was significantly dampened by 2-Cl-Phen. However, cotreatment with a proteasome inhibitor, MG132, restored Herp expression only to a limited extent. Taken together, these results show that 2-Cl-Phen changed the expression of several ERAD components, especially by transcriptional inhibition of Herp induction by 2-Cl-Phen when it occurred at an early phase, and this finding provides new insights into understanding the mechanisms of 2-Cl-Phen-mediated cytotoxicity.




Similar content being viewed by others
Abbreviations
- ATF4:
-
Activating transcription factor 4
- ATF6:
-
Activating transcription factor 6
- 2-Cl-Phen:
-
2-(2-Chlorophenyl)ethylbiguanide
- CHX:
-
Cycloheximide
- Derlin-1:
-
Degradation in endoplasmic reticulum protein 1
- ER:
-
Endoplasmic reticulum
- ERAD:
-
ER stress-associated protein degradation
- GRP78:
-
78 kDa glucose-regulated protein
- GRP94:
-
94 kDa glucose-regulated protein
- G3PDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- Herp:
-
Homocysteine-induced ER protein
- Hrd1:
-
Hydroxymethylglutaryl reductase degradation 1
- OS9:
-
Osteosarcoma amplified 9
- PERK:
-
PKR-like endoplasmic reticulum kinase
- PDI:
-
Protein disulfide isomerase
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SEL1L:
-
Suppressor/enhancer of lin-12-like
- VCP:
-
Valosin-containing protein
References
Helenius, A., Marquardt, T., & Braakman, I. (1992). The endoplasmic reticulum as protein-folding compartment. Trends in Cell Biology, 2(8), 227–231.
Zhu, C., Johansen, F. E., & Prywes, R. (1997). Interaction of ATF6 and serum response factor. Molecular and Cellular Biology, 17(9), 4957–4966.
Calfon, M., Zeng, H., Urano, F., Till, J. H., Hubbard, S. R., Harding, H. P., Clark, S. G., & Ron, D. (2002). IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature, 415(6867), 92–96.
Harding, H. P., Zhang, Y., & Ron, D. (1999). Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature, 397(6716), 271–274.
Kim, I., Xu, W., & Reed, J. C. (2008). Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nature Reviews Drug Discovery, 7(12), 1013–1030.
Cao, S. S., & Kaufman, R. J. (2014). Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants & Redox Signaling, 21(3), 396–413.
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T., & Mori, K. (2001). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell, 107(7), 881–891.
Lee, A. H., Iwakoshi, N. N., & Glimcher, L. H. (2003). XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Molecular and Cellular Biology, 23(21), 7448–7459.
Okada, T., Yoshida, H., Akazawa, R., Negishi, M., & Mori, K. (2002). Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. The Biochemical Journal, 366(2), 585–594.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674.
Fu, Y., & Lee, A. S. (2006). Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biology & Therapy, 5(7), 741–744.
Bi, M., Naczki, C., Koritzinsky, M., Fels, D., Blais, J., Hu, N., Harding, H., Novoa, I., Varia, M., Raleigh, J., Scheuner, D., Kaufman, R. J., Bell, J., Ron, D., Wouters, B. G., & Koumenis, C. (2005). ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. The EMBO Journal, 24(19), 3470–3481.
Pyrko, P., Schonthal, A. H., Hofman, F. M., Chen, T. C., & Lee, A. S. (2007). The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Research, 67(20), 9809–9816.
Narise, K., Okuda, K., Enomoto, Y., Hirayama, T., & Nagasawa, H. (2014). Optimization of biguanide derivatives as selective antitumor agents blocking adaptive stress responses in the tumor microenvironment. Drug Design, Development and Therapy, 8, 701–717.
Oh-hashi K, Irie N, Sakai T, Okuda K, Nagasawa H, Hirata Y. Kiuchi K. (2016) Elucidation of a novel phenformin derivative on glucose-deprived stress responses in HT-29 cells. Molecular and Cellular Biochemistry, 419(1-2), 29–40.
Oh-hashi, K., Matsumoto, S., Sakai, T., Nomura, Y., Okuda, K., Nagasawa, H., & Hirata, Y. (2018). Elucidating the rapid action of 2-(2-chlorophenyl) ethylbiguanide on HT-29 cells under a serum- and glucose-deprived condition. Cell Biology and Toxicology, 34(4), 279–290.
Hoseki, J., Ushioda, R., & Nagata, K. (2010). Mechanism and components of endoplasmic reticulum associated degradation. Journal of Biochemistry, 147(1), 19–25.
Wang, Q., Mora-Jensen, H., Weniger, M. A., Perez-Galan, P., Wolford, C., Hai, T., Ron, D., Chen, W., Trenkle, W., Wiestner, A., & Ye, Y. (2009). ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 106(7), 2200–2205.
Chou, T. F., Li, K., Frankowski, K. J., Schoenen, F. J., & Deshaies, R. J. (2013). Structure-activity relationship study reveals ML240 and ML241 as potent and selective inhibitors of p97 ATPase. ChemMedChem, 8(2), 297–312.
Norisada, J., Hirata, Y., Amaya, F., Kiuchi, K., & Oh-hashi, K. (2016). A comparative analysis of the molecular features of MANF and CDNF. PLoS One, 11(1), e0146923.
Kokame, K., Agarwala, K. L., Kato, H., & Miyata, T. (2000). Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. The Journal of Biological Chemistry, 275(42), 32846–32853.
Ma, Y., & Hendershot, L. M. (2004). Herp is dually regulated by both the endoplasmic reticulum stress-specific branch of the unfolded protein response and a branch that is shared with other cellular stress pathways. The Journal of Biological Chemistry, 279(14), 13792–13799.
Chan, S. L., Fu, W., Zhang, P., Cheng, A., Lee, J., Kokame, K., & Mattson, M. P. (2004). Herp stabilizes neuronal Ca2+ homeostasis and mitochondrial function during endoplasmic reticulum stress. The Journal of Biological Chemistry, 279(27), 28733–28743.
Kaneko, M., Yasui, S., Niinuma, Y., Arai, K., Omura, T., Okuma, Y., & Nomura, Y. (2007). A different pathway in the endoplasmic reticulum stress-induced expression of human HRD1 and SEL1 genes. FEBS Letters, 581(28), 5355–5360.
Iida, Y., Fujimori, T., Okawa, K., Nagata, K., Wada, I., & Hosokawa, N. (2011). SEL1L protein critically determines the stability of the HRD1-SEL1L endoplasmic reticulum-associated degradation (ERAD) complex to optimize the degradation kinetics of ERAD substrates. The Journal of Biological Chemistry, 286(19), 16929–16939.
Bernasconi, R., Pertel, T., Luban, J., & Molinari, M. (2008). A dual task for the Xbp1-responsive OS-9 variants in the mammalian endoplasmic reticulum: Inhibiting secretion of misfolded protein conformers and enhancing their disposal. The Journal of Biological Chemistry, 283(24), 16446–16454.
Shen, J., Chen, X., Hendershot, L., & Prywes, R. (2002). ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Developmental Cell, 3(1), 99–111.
Sou, S. N., Ilieva, K. M., & Polizzi, K. M. (2012). Binding of human BiP to the ER stress transducers IRE1 and PERK requires ATP. Biochemical and Biophysical Research Communications, 420(2), 473–478.
Christianson, J. C., Shaler, T. A., Tyler, R. E., & Kopito, R. R. (2008). OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nature Cell Biology, 10(3), 272–282.
Nakamura, N., Rabouille, C., Watson, R., Nilsson, T., Hui, N., Slusarewicz, P., Kreis, T. E., & Warren, G. (1995). Characterization of a cis-Golgi matrix protein, GM130. The Journal of Cell Biology, 131(6), 1715–1726.
Reiling JH, Olive AJ, Sanyal S, Carette JE, Brummelkamp TR, Ploegh HL, Starnbach MN, Sabatini,DM. (2013) A CREB3-ARF4 signalling pathway mediates the response to Golgi stress and susceptibility to pathogens. Nature Cell Biology 15(12), 1473–1485.
Taniguchi, M., & Yoshida, H. (2017). TFE3, HSP47, and CREB3 pathways of the mammalian Golgi stress response. Cell Structure and Function, 42(1), 27–36.
Oh-hashi, K., Soga, A., Naruse, Y., Takahashi, K., Kiuchi, K., & Hirata, Y. (2018). Elucidating post-translational regulation of mouse CREB3 in Neuro2a cells. Molecular and Cellular Biochemistry, 448(1-2), 287–297.
Yamamoto, K., Suzuki, N., Wada, T., Okada, T., Yoshida, H., Kaufman, R. J., & Mori, K. (2008). Human HRD1 promoter carries a functional unfolded protein response element to which XBP1 but not ATF6 directly binds. Journal of Biochemistry, 144(4), 477–486.
Sai, X., Kokame, K., Shiraishi, H., Kawamura, Y., Miyata, T., Yanagisawa, K., & Komano, H. (2003). The ubiquitin-like domain of Herp is involved in Herp degradation, but not necessary for its enhancement of amyloid beta-protein generation. FEBS Letters, 553(1-2), 151–156.
Tanaka, T., Tsujimura, T., Takeda, K., Sugihara, A., Maekawa, A., Terada, N., Yoshida, N., & Akira, S. (1998). Targeted disruption of ATF4 discloses its essential role in the formation of eye lens fibres. Genes to Cells, 3(12), 801–810.
Masuoka, H. C., & Townes, T. M. (2002). Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood, 99(3), 736–745.
Pasini, S., Corona, C., Liu, J., Greene, L. A., & Shelanski, M. L. (2015). Specific downregulation of hippocampal ATF4 reveals a necessary role in synaptic plasticity and memory. Cell Reports, 11(2), 183–191.
Miura, H., Hashida, K., Sudo, H., Awa, Y., Takarada-Iemata, M., Kokame, K., Takahashi, T., Matsumoto, M., Kitao, Y., & Hori, O. (2010). Deletion of Herp facilitates degradation of cytosolic proteins. Genes to Cells, 15(8), 843–853.
Eura, Y., Yanamoto, H., Arai, Y., Okuda, T., Miyata, T., & Kokame, K. (2012). Derlin-1 deficiency is embryonic lethal, Derlin-3 deficiency appears normal, and Herp deficiency is intolerant to glucose load and ischemia in mice. PLoS One, 7(3), e34298.
Sun S, Shi G, Han X, Francisco AB, Ji Y, Mendonça N, Liu X, Locasale JW, Simpson KW, Duhamel GE, Kersten S, Yates JR 3rd, Long Q, Qi L. (2014) Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival. Proceedings of the National Academy of Sciences of the United States of America 111(5), E582–E591
Oyadomari, S., & Mori, M. (2004). Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death and Differentiation, 11(4), 381–389.
Lee, C. Y., Lee, M. G., Choi, K. C., Kang, H. M., & Chang, Y. S. (2012). Clinical significance of GADD153 expression in stage I non-small cell lung cancer. Oncology Letters, 4(3), 408–412.
Thevenot, P. T., Sierra, R. A., Raber, P. L., Al-Khami, A. A., Trillo-Tinoco, J., Zarreii, P., Ochoa, A. C., Cui, Y., Del Valle, L., & Rodriguez, P. C. (2014). The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity, 41(3), 389–401.
Liu, Z., Zhang, Q., Peng, H., & Zhang, W. Z. (2012). Animal lectins: potential antitumor therapeutic targets in apoptosis. Applied Biochemistry and Biotechnology, 168(3), 629–637.
Song, W., Yang, H. B., Chen, P., Wang, S. M., Zhao, L. P., Xu, W. H., Fan, H. F., Gu, X., & Chen, L. Y. (2013). Apoptosis of human gastric carcinoma SGC-7901 induced by deoxycholic acid via the mitochondrial-dependent pathway. Applied Biochemistry and Biotechnology, 171(4), 1061–1071.
Funding
This work is partly supported by the Koshiyama Science and Technology Foundation (to Kensuke Okuda), Grant-in-Aid for Challenging Exploratory Research (no. 17K19901 to Kentaro Oh-hashi), and the OGAWA Science and Technology Foundation (to Kentaro Oh-hashi).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
Effect of tunicamycin on OS9 protein expression in HT-29 cells. HT-29 cells were incubated in serum-containing medium together with or without tunicamycin (Tm, 1 μg/ml) or serum- and glucose-deprived medium for 24 h. The expression levels of the indicated protein were determined as described in the “Materials and methods” section. (PPTX 54.4 kb)
Supplementary Fig. 2
Effect of MG132 and cycloheximide on ATF4 protein expression in HT-29 cells under a serum- and glucose-deprived condition. HT-29 cells were incubated in the serum-containing or serum- and glucose-deprived medium together with 2-Cl-Phen (50 μM), cycloheximide (CHX, 10 μg/ml), MG132 (MG, 10 μM), or vehicle for 12 h. The expression levels of the indicated protein were determined as described in the “Materials and methods” section. Representative results are shown in this figure. (PPTX 180 kb)
Rights and permissions
About this article
Cite this article
Oh-hashi, K., Matsumoto, S., Sakai, T. et al. Effects of 2-(2-Chlorophenyl)ethylbiguanide on ERAD Component Expression in HT-29 Cells Under a Serum- and Glucose-Deprived Condition. Appl Biochem Biotechnol 188, 1009–1021 (2019). https://doi.org/10.1007/s12010-019-02969-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-02969-4