Abstract
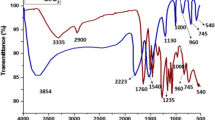
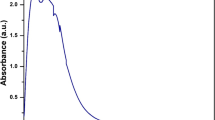
In the present studies, renewable and nontoxic biopolymer, pectin, was extracted from Indian red pomelo fruit peels and used for the synthesis of cerium oxide nanoparticles (CeO2-NPs) having bio-therapeutic potential. The structural information of extracted pectin was investigated by FTIR and NMR spectroscopic techniques. Physicochemical characteristics of this pectin suggested its application in the synthesis of metal oxide nanoparticles. Using this pectin as a template, CeO2-NPs were synthesized by simple, one step and eco-friendly approach. The UV–Vis spectrum of synthesized CeO2-NPs exhibited a characteristic absorption peak at wavelength 345 nm, which can be assigned to its intrinsic band gap (3.59 eV) absorption. Photoluminescence measurements of CeO2-NPs revealed that the broad emission was composed of seven different bands. FTIR analysis ensured involvement of pectin in the formation and stabilization of CeO2-NPs. FT-Raman spectra showed a sharp Raman active mode peak at 461.8 cm−1 due to a symmetrical stretching mode of Ce–O vibration. DLS, FESEM, EDX, and XRD analysis showed that the CeO2-NPs prepared were polydispersed, spherical shaped with a cubic fluorite structure and average particle size ≤40 nm. These CeO2-NPs displayed broad spectrum antimicrobial activity, antioxidant potential, and non-cytotoxic nature.








Similar content being viewed by others
References
Kockrick, E., Schrage, C., Grigas, A., Geiger, D., & Kaskel, S. (2008). Synthesis and catalytic properties of microemulsion-derived cerium oxide nanoparticles. Journal of Solid State Chemistry, 181(7), 1614–1620.
Selvan, V. A. M., Anand, R. B., & Udayakumar, M. (2009). Effects of cerium oxide nanoparticle addition in diesel and diesel–biodiesel–ethanol blends on the performance and emission characteristics of a CI engine. Journal of Engineering and Applied Science, 4(7), 1819–6608.
Jacobs, G., Williams, L., Graham, U., Thomas, G. A., Sparks, D. E., & Davis, B. H. (2003). Low temperature water–gas shift: in situ DRIFTS-reaction study of ceria surface area on the evolution of formates on Pt/CeO2 fuel processing catalysts for fuel cell applications. Applied Catalysis A: General, 252(1), 107–118.
Kaittanis, C., Santra, S., Asati, A., & Perez, J. M. (2012). A cerium oxide nanoparticles based device for the detection of chronic inflammation via optical and magnetic resonance imaging. Nanoscale, 4(6), 2117–2123.
Khan, S. B., Faisal, M., Rahman, M. M., & Jamal, A. (2011). Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications. Science of the Total Environment, 409(15), 2987–2992.
Yabe, S., & Sato, T. (2003). Cerium oxide for sunscreen cosmetics. Journal of Solid State Chemistry, 171(1), 7–11.
Celardo, I., Pedersen, J. Z., Traversa, E., & Ghibelli, L. (2011). Pharmacological potential of cerium oxide nanoparticles. Nanoscale, 3(4), 1411–1420.
Das, S., Dowding, J. M., Klump, K. E., McGinnis, J. F., Self, W., & Seal, S. (2013). Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine, 8(9), 1483–1508.
Soren, S., Jena, S. R., Samanta, L., & Parhi, P. (2015). Antioxidant potential and toxicity study of the cerium oxide nanoparticles synthesized by microwave-mediated synthesis. Applied Biochemistry and Biotechnology, 177(1), 148–161.
Sun, C., Li, H., & Chen, L. (2012). Nanostructured ceria-based materials: synthesis, properties, and applications. Energy & Environmental Science, 5(9), 8475–8505.
Arumugam, A., Karthikeyan, C., Hameed, A. S. H., Gopinath, K., Gowri, S., & Karthika, V. (2015). Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Materials Science and Engineering: C, 49, 408–415.
Kargar, H., Ghasemi, F., & Darroudi, M. (2015). Bioorganic polymer-based synthesis of cerium oxide nanoparticles and their cell viability assays. Ceramics International, 41(1), 1589–1594.
Darroudi, M., Sarani, M., Oskuee, R. K., Zak, A. K., Hosseini, H. A., & Gholami, L. (2014). Green synthesis and evaluation of metabolic activity of starch mediated nanoceria. Ceramics International, 40(1), 2041–2045.
Sifontes, A. B., Gonzalez, G., Ochoa, J. L., Tovar, L. M., Zoltan, T., & Canizales, E. (2011). Chitosan as template for the synthesis of ceria nanoparticles. Materials Research Bulletin, 46(11), 1794–1799.
Darroudi, M., Hakimi, M., Sarani, M., Oskuee, R. K., Zak, A. K., & Gholami, L. (2013). Facile synthesis, characterization, and evaluation of neurotoxicity effect of cerium oxide nanoparticles. Ceramics International, 39(6), 6917–6921.
Darroudi, M., Sarani, M., Oskuee, R. K., Zak, A. K., & Amiri, M. S. (2014). Nanoceria: gum mediated synthesis and in vitro viability assay. Ceramics International, 40(2), 2863–2868.
Rolin, C. (2002). 8 Commercial pectin preparations. In G. B. Seymour & J. P. Knox (Eds.), Pectins and their manipulation (1st ed., pp. 222–223). Oxford: Blackwell.
Rangel-Rodríguez, A. M., Conxita, S., Susana, V., Flores-Gallardo, S. G., Contreras-Esquivel, J. C., & Licea-Jiménez, L. (2014). Immobilization of pectinesterase in genipin-crosslinked chitosan membrane for low methoxyl pectin production. Applied Biochemistry and Biotechnology, 174(8), 2941–2950.
Zahran, M. K., Ahmed, H. B., & El-Rafie, M. H. (2014). Facile size-regulated synthesis of silver nanoparticles using pectin. Carbohydrate Polymers, 111, 971–978.
Nigoghossian, K., dos Santos, M. V., Barud, H. S., da Silva, R. R., Rocha, L. A., Caiut, J. M., de Assuncao, R. M. N., Spanhele, L., Poulain, M., Messaddeq, Y., & Ribeiro, S. J. (2015). Orange pectin mediated growth and stability of aqueous gold and silver nanocolloids. Applied Surface Science, 341, 28–36.
Wang, A. J., Liao, Q. C., Feng, J. J., Zhang, P. P., Li, A. Q., & Wang, J. J. (2012). Apple pectin-mediated green synthesis of hollow double-caged peanut-like ZnO hierarchical superstructures and photocatalytic applications. CrystEngComm, 14(1), 256–263.
Gupta, V. K., Pathania, D., Agarwal, S., & Singh, P. (2012). Adsorptional photocatalytic degradation of methylene blue onto pectin–CuS nanocomposite under solar light. Journal of Hazardous Materials, 243, 179–186.
Gopi, D., Kanimozhi, K., Bhuvaneshwari, N., Indira, J., & Kavitha, L. (2014). Novel banana peel pectin mediated green route for the synthesis of hydroxyapatite nanoparticles and their spectral characterization. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 118, 589–597.
Gopi, D., Kanimozhi, K., & Kavitha, L. (2015). Opuntia ficus indica peel derived pectin mediated hydroxyapatite nanoparticles: synthesis, spectral characterization, biological and antimicrobial activities. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 141, 135–143.
Kravtchenko, T. P., Voragen, A. G. J., & Pilnik, W. (1992). Analytical comparison of three industrial pectin preparations. Carbohydrate Polymers, 18(1), 17–25.
Online: Crop Info and How-To Guide in Growing Pummelo. Available from: www.cropsreview.com/pummelo.html. Accessed 21 Apr 2016.
Online: C I T R U S: Statistics - fresh and processed citrus fruit. Available from: www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Citrus/Documents/CITRUS_BULLETIN_2012.pdf. Accessed 21 Apr 2016.
Bagherian, H., Ashtiani, F. Z., Fouladitajar, A., & Mohtashamy, M. (2011). Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chemical Engineering and Processing: Process Intensification, 50(11), 1237–1243.
Goel, H., Kaur, G., Tiwary, A. K., & Rana, V. (2010). Formulation development of stronger and quick disintegrating tablets: a crucial effect of chitin. Yakugaku Zasshi, 130(5), 729–735.
Jindal, M., Kumar, V., Rana, V., & Tiwary, A. K. (2013). Aegle marmelos fruit pectin for food and pharmaceuticals: physico-chemical, rheological and functional performance. Carbohydrate Polymers, 93(2), 386–394.
Arslan, N. (1995). Extraction of pectin from sugar-beet pulp and intrinsic viscosity molecular weight relationship of pectin solutions. Journal of Food Science and Technology-Mysore, 32(5), 381–385.
Das, D., Nath, B. C., Phukon, P., & Dolui, S. K. (2013). Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids and Surfaces B: Biointerfaces, 111, 556–560.
Lu, Z., Mao, C., Meng, M., Liu, S., Tian, Y., Yu, L., Sun, B., & Li, C. M. (2014). Fabrication of CeO2 nanoparticle-modified silk for UV protection and antibacterial applications. Journal of Colloid and Interface Science, 435, 8–14.
Kumar, K. P., Paul, W., & Sharma, C. P. (2011). Green synthesis of gold nanoparticles with Zingiber officinale extract: characterization and blood compatibility. Process Biochemistry, 46(10), 2007–2013.
Visser, J., & Voragen, A. G. J. (Eds.). (1996). Pectins and pectinases. In Progress in Biotechnology (pp. 3–19). Amsterdam: Elsevier Science.
Da Silva, J. L., & Rao, M. A. (2006). 11 pectins: structure, functionality, and uses. In A. M. Stephen, G. O. Phillips, & P. A. Williams (Eds.), Food polysaccharides and their applications (2nd ed., pp. 353–397). New York: CRC Press.
Tamaki, Y., Uechi, S., Taira, T., Ishihara, M., Adaniya, S., Uesato, K., Fukuda, M., & Tako, M. (2004). Isolation and characterization of pectin from pericarp of Citrus depressa. Journal of Applied Glycoscience, 51(1), 19–25.
Habibi, Y., Heyraud, A., Mahrouz, M., & Vignon, M. R. (2004). Structural features of pectic polysaccharides from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydrate Research, 339(6), 1119–1127.
Ivanova, N. V., Trofimova, N. N., Es’kova, L. A., & Babkin, V. A. (2012). The study of the reaction of Pectin-Ag (0) nanocomposites formation. International Journal of Carbohydrate Chemistry. doi:10.1155/2012/459410.
Zak, A. K., Majid, W. A., Mahmoudian, M. R., Darroudi, M., & Yousefi, R. (2013). Starch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties study. Advanced Powder Technology, 24(3), 618–624.
Sohnel, O., & Garside, J. (1992). Precipitation: Basic principles and industrial applications. Butterworth-Heinemann.
Chen, H. I., & Chang, H. Y. (2004). Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 242(1), 61–69.
Orel, Z. C., & Orel, B. (1994). Optical properties of pure CeO2 and mixed CeO2/SnO2 thin film coatings. Physica Status Solidi B, 186(1), K33–K36.
Tsunekawa, S., Wang, J. T., Kawazoe, Y., & Kasuya, A. (2003). Blueshifts in the ultraviolet absorption spectra of cerium oxide nanocrystallites. Journal of Applied Physics, 94(5), 3654–3656.
Wang, L., Ren, J., Liu, X., Lu, G., & Wang, Y. (2011). Evolution of SnO2 nanoparticles into 3D nanoflowers through crystal growth in aqueous solution and its optical properties. Materials Chemistry and Physics, 127(1), 114–119.
Sun, C., Li, H., Zhang, H., Wang, Z., & Chen, L. (2005). Controlled synthesis of CeO2 nanorods by a solvothermal method. Nanotechnology, 16(9), 1454.
Wang, G., Mu, Q., Chen, T., & Wang, Y. (2010). Synthesis, characterization and photoluminescence of CeO2 nanoparticles by a facile method at room temperature. Journal of Alloys and Compounds, 493(1), 202–207.
George, S., Pokhrel, S., Xia, T., Gilbert, B., Ji, Z., Schowalter, M., Rosenauer, A., Damoiseaux, R., Bradley, K. A., Mädler, L., & Nel, A. E. (2009). Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano, 4(1), 15–29.
Wang, A. Q., D’Souza, N., & Golden, T. D. (2006). Electrosynthesis of nanocrystalline cerium oxide/layered silicate powders. Journal of Materials Chemistry, 16(5), 481–488.
Darroudi, M., Hoseini, S. J., Oskuee, R. K., Hosseini, H. A., Gholami, L., & Gerayli, S. (2014). Food-directed synthesis of cerium oxide nanoparticles and their neurotoxicity effects. Ceramics International, 40(5), 7425–7430.
Khan, S. B., Faisal, M., Rahman, M. M., Akhtar, K., Asiri, A. M., Khan, A., & Alamry, K. A. (2013). Effect of particle size on the photocatalytic activity and sensing properties of CeO2 nanoparticles. International Journal of Electrochemical Science, 8, 7284–7297.
Korsvik, C., Patil, S., Seal, S., & Self, W. T. (2007). Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chemical Communications, 14(10), 1056–1058.
Kuang, Y., He, X., Zhang, Z., Li, Y., Zhang, H., Ma, Y., Wu, Z., & Chai, Z. (2011). Comparison study on the antibacterial activity of nano-or bulk-cerium oxide. Journal of Nanoscience and Nanotechnology, 11(5), 4103–4108.
Gopinath, K., Karthika, V., Sundaravadivelan, C., Gowri, S., & Arumugam, A. (2015). Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities. Journal of Nanostructure in Chemistry, 5(3), 295–303.
Stoimenov, P. K., Klinger, R. L., Marchin, G. L., & Klabunde, K. J. (2002). Metal oxide nanoparticles as bactericidal agents. Langmuir, 18(17), 6679–6686.
Tong, G. X., Du, F. F., Liang, Y., Hu, Q., Wu, R. N., Guan, J. G., & Hu, X. (2013). Polymorphous ZnO complex architectures: selective synthesis, mechanism, surface area and Zn-polar plane-codetermining antibacterial activity. Journal of Materials Chemistry B, 1(4), 454–463.
Hajipour, M. J., Fromm, K. M., Ashkarran, A. A., de Aberasturi, D. J., de Larramendi, I. R., Rojo, T., Serpooshan, V., Parak, W. J., & Mahmoudi, M. (2012). Antibacterial properties of nanoparticles. Trends in Biotechnology, 30(10), 499–511.
Sharp, M. K., & Mohammad, S. F. (1998). Scaling of hemolysis in needles and catheters. Annals of Biomedical Engineering, 26(5), 788–797.
Singhal, J. P., & Ray, A. R. (2002). Synthesis of blood compatible polyamide block copolymers. Biomaterials, 23(4), 1139–1145.
Acknowledgment
SNP and JSP are thankful to the UGC, New Delhi for UGS-BSR/RFSMS fellowships. PBC is grateful to the DST, New Delhi for INSPIRE fellowship. All the authors acknowledge the SAIF-Chandigarh for NMR spectroscopic analysis, SAIF-IIT Chennai for FT-Raman spectroscopic study and UGS-SAP-DRS III and DST-FIST for providing infrastructure at School of Life Sciences of North Maharashtra University, Jalgaon.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 866 kb)
Rights and permissions
About this article
Cite this article
Patil, S.N., Paradeshi, J.S., Chaudhari, P.B. et al. Bio-therapeutic Potential and Cytotoxicity Assessment of Pectin-Mediated Synthesized Nanostructured Cerium Oxide. Appl Biochem Biotechnol 180, 638–654 (2016). https://doi.org/10.1007/s12010-016-2121-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2121-9