Abstract
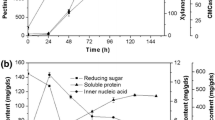
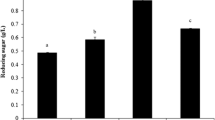
The effects of surfactants and microwave pretreatment of orange peel powder (OPP) on the production of pectinase, cellulase, and xylanase by Aspergillus japonicus PJ01 in submerged fermentation were investigated. The results showed that when OPP was pretreated only by microwave (630 W, 9 min, and liquid/solid ratio 5), the activities of exo-pectinase, carboxymethyl cellulase (CMCase), xylanase, and filter paper cellulase (FPase) were increased by 11.8, 20.6, 16.2, and 24.0 %, respectively, and when OPP was pretreated by microwave at the same conditions cited above plus PEG 4000 at the concentration of 3 g/L, the activities of the above four enzymes were enhanced by 40.2, 30.3, 40.4, and 40.0 % after 84-h cultivation, respectively. It is suggested a synergistic effect between microwave and surfactant treatment in enhancing the multiextracellular enzymes production by OPP fermentation of A. japonicus PJ01. Chemical composition and Fourier transform infrared spectroscopy (FTIR) analysis displayed that the microwave pretreatment of OPP led to the decrease of hemicellulose and essential oil contents. Scanning electron microscope (SEM) showed that OPP surface after microwave pretreatment became porous and more susceptible to be invaded by A. japonicus. The results demonstrated that pretreatment of OPP by surfactant PEG 4000 and microwave irradiation as environment-friendly way was cost-effective in enhancing the multienzyme production from agricultural waste orange peel.





Similar content being viewed by others
References
Pedrolli, D. B., Gomes, E., Monti, R., & Carmona, E. C. (2008). Studies on productivity and characterization of polygalacturonase from Aspergillus giganteus submerged culture using citrus pectin and orange waste. Applied Biochemistry and Biotechnology, 144, 191–200. doi:10.1007/s12010-014-1143-4.
Woldesenbet, F., Virk, A. P., Gupta, N., & Sharma, P. (2012). Effect of microwave irradiation on xylanase production from wheat bran and biobleaching of eucalyptus kraft pulp. Applied Biochemistry and Biotechnology, 167, 100–108. doi:10.1007/s12010-012-9663-2.
Ismail, A.-M. S. (1996). Utilization of orange peels for the production of multienzyme complexes by some fungal strains. Process Biochemistry, 31, 645–650. doi:10.1016/S0032-9592(96)00012-X.
Kumar, S., Sharma, H., & Sarkar, B. (2011). Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged (SmF) and solid state fermentation (SSF). Food Science and Biotechnology, 20, 1289–1298. doi:10.1007/s10068-011-0178-3.
Sharma, S., Mandhan, R. P., & Sharma, J. (2012). Utilization of agro-industrial residues for pectinase production by the novel strain Pseudozyma sp. SPJ under solid state cultivation. Annals of Microbiology, 62, 169–176. doi:10.1007/s13213-011-0243-4.
Hu, H. L., van den Brink, J., Gruben, B. S., Wösten, H. A. B., Gu, J. D., & de Vries, R. P. (2011). Improved enzyme production by co-cultivation of Aspergillus niger and Aspergillus oryzae and with other fungi. International Biodeterioration & Biodegradation, 65, 248–252. doi:10.1016/j.ibiod.2010.11.008.
Sharma, D., & Satyanarayana, T. (2006). A marked enhancement in the production of a highly alkaline and thermostable pectinase by Bacillus pumilus dcsr1 in submerged fermentation by using statistical methods. Bioresource Technology, 97, 727–733. doi:10.1016/j.biortech.2005.04.012.
Gonçalves, D., Teixeira, J. A., Bazzolli, D. S., de Queiroz, M., & de Araújo, E. (2012). Use of response surface methodology to optimize production of pectinases by recombinant Penicillium griseoroseum T20. Biocatalysis and Agricultural Biotechnology, 1, 140–146. doi:10.1016/j.bcab.2011.09.002.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y., Holtzapple, M., & Ladisch, M. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96, 673–686. doi:10.1016/j.biortech.2004.06.025.
Sun, Y., & Cheng, J. (2002). Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresource Technology, 83, 1–11. doi:10.1016/S0960-8524(01)00212-7.
Singh, A., & Bishnoi, N. R. (2012). Enzymatic hydrolysis optimization of microwave alkali pretreated wheat straw and ethanol production by yeast. Bioresource Technology, 108, 94–101. doi:10.1016/j.biortech.2011.12.084.
Boonsombuti, A., Luengnaruemitchai, A., & Wongkasemjit, S. (2013). Enhancement of enzymatic hydrolysis of corncob by microwave-assisted alkali pretreatment and its effect in morphology. Cellulose, 20, 1957–1966. doi:10.1007/s10570-013-9958-7.
Pang, F., Xue, S., Yu, S., Zhang, C., Li, B., & Kang, Y. (2013). Effects of combination of steam explosion and microwave irradiation (SE–MI) pretreatment on enzymatic hydrolysis, sugar yields and structural properties of corn stover. Industrial Crops and Products, 42, 402–408. doi:10.1016/j.indcrop.2012.06.016.
Peng, H., Li, H., Luo, H., & Xu, J. (2013). A novel combined pretreatment of ball milling and microwave irradiation for enhancing enzymatic hydrolysis of microcrystalline cellulose. Bioresource Technology, 130, 81–87. doi:10.1016/j.biortech.2012.10.167.
Pardo, A. G. (1996). Effect of surfactants on cellulase production by Nectria catalinensis. Current Microbiology, 33, 275–278. doi:10.1007/s002849900113.
Reddy, R. M., Reddy, P. G., & Seenayya, G. (1999). Enhanced production of thermostable β-amylase and pullulanase in the presence of surfactants by Clostridium thermosulfurogenes SV2. Process Biochemistry, 34, 87–92. doi:10.1016/S0032-9592(98)00073-9.
Zeng, G.-M., Shi, J.-G., Yuan, X.-Z., Liu, J., Zhang, Z.-B., Huang, G.-H., Li, J.-B., Xi, B.-D., & Liu, H.-L. (2006). Effects of Tween 80 and rhamnolipid on the extracellular enzymes of Penicillium simplicissimum isolated from compost. Enzyme and Microbial Technology, 39, 1451–1456. doi:10.1016/j.enzmictec.2006.03.035.
Liu, X.-L., Zeng, G.-M., Tang, L., Zhong, H., Wang, R.-Y., Fu, H.-Y., Liu, Z.-F., Huang, H.-L., & Zhang, J.-C. (2008). Effects of dirhamnolipid and SDS on enzyme production from Phanerochaete chrysosporium in submerged fermentation. Process Biochemistry, 43, 1300–1303. doi:10.1016/j.procbio.2008.06.007.
Teather, R. M., & Wood, P. J. (1982). Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Applied and Environmental Microbiology, 43, 777–780.
Liu, G., Xu, Z., & Cen, P. (2000). A morphologically structured model for mycelial growth and secondary metabolite formation. Chinese Journal of Chemical Engineering, 8, 46–51.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry, 72, 248–254.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428. doi:10.1021/ac60147a030.
Díaz, A. B., Bolívar, J., de Ory, I., Caro, I., & Blandino, A. (2011). Applicability of enzymatic extracts obtained by solid state fermentation on grape pomace and orange peels mixtures in must clarification. LWT - Food Science and Technology, 44, 840–846. doi:10.1016/j.lwt.2010.12.006.
Ghose, T. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268. doi:10.1351/pac198759020257.
Bailey, M. J., Biely, P., & Poutanen, K. (1992). Interlaboratory testing of methods for assay of xylanase activity. Journal of Biotechnology, 23, 257–270. doi:10.1016/0168-1656(92)90074-J.
Liu, S. (2004). Analysis and measurement in papermaking industry. Beijing: China Chemical Industry Press.
Charles, D. J., & Simon, J. E. (1990). Comparison of extraction methods for the rapid determination of essential oil content and composition of basil. Journal of the American Society for Horticultural Science, 115, 458–462.
Ahuja, S., Ferreira, G., & Moreira, A. (2004). Production of an endoglucanase by the shipworm bacterium, Teredinobacter turnirae. Journal of Industrial Microbiology & Biotechnology, 31, 41–47. doi:10.1007/s10295-004-0113-1.
Ma, H., Liu, W.-W., Chen, X., Wu, Y.-J., & Yu, Z.-L. (2009). Enhanced enzymatic saccharification of rice straw by microwave pretreatment. Bioresource Technology, 100, 1279–1284. doi:10.1016/j.biortech.2008.08.045.
Wilkins, M. R., Widmer, W. W., & Grohmann, K. (2007). Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochemistry, 42, 1614–1619. doi:10.1016/j.procbio.2007.09.006.
Gnanasambandam, R., & Proctor, A. (2000). Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chemistry, 68, 327–332. doi:10.1016/S0308-8146(99)00191-0.
Li, F. T., Yang, H., Zhao, Y., & Xu, R. (2007). Novel modified pectin for heavy metal adsorption. Chinese Chemical Letters, 18, 325–328. doi:10.1016/j.cclet.2007.01.034.
Hsu, T.-C., Guo, G.-L., Chen, W.-H., & Hwang, W.-S. (2010). Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresource Technology, 101, 4907–4913. doi:10.1016/j.biortech.2009.10.009.
Guo, G.-L., Chen, W.-H., Chen, W.-H., Men, L.-C., & Hwang, W.-S. (2008). Characterization of dilute acid pretreatment of silvergrass for ethanol production. Bioresource Technology, 99, 6046–6053. doi:10.1016/j.biortech.2007.12.047.
Zhao, X., Zhou, Y., Zheng, G., & Liu, D. (2010). Microwave pretreatment of substrates for cellulase production by solid-state fermentation. Applied Biochemistry and Biotechnology, 160, 1557–1571. doi:10.1007/s12010-009-8640-x.
Acknowledgments
This work was supported by a grant from the National High Technology Research and Development Program of China (863 Program) (No. 2011AA100804), Hunan Provincial Innovation Foundation for Postgraduate (CX2013B084), and Open-End Fund for the Valuable and Precision Instruments of Central South University (CSUZC2014003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Pj., Xia, Jl., Shan, Y. et al. Effects of Surfactants and Microwave-assisted Pretreatment of Orange Peel on Extracellular Enzymes Production by Aspergillus japonicus PJ01. Appl Biochem Biotechnol 176, 758–771 (2015). https://doi.org/10.1007/s12010-015-1609-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1609-z