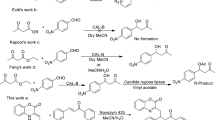
Abstract
Enzymatic regioselective acylation of 5-azacytidine with vinyl laurate was successfully conducted with an immobilized lipase from Candida antarctica type B (i.e., Novozym 435) for the first time. The acylation of 5-azacytidine took place at its primary hydroxyl group and the desired product 5′-O -lauroyl-5-azacytidine could be prepared with high reaction rate, high conversion, and excellent regioselectivity. The influences of several key variables on the enzymatic acylation were also systematically examined. Pyridine was found to be the best reaction medium. The optimum initial water activity, the molar ratio of vinyl laurate to 5-azacytidine and reaction temperature were 0.07, 30:1, and 50 °C, respectively. Under the optimized conditions described above, the initial reaction rate, the substrate conversion, and the regioselectivity were as high as 0.58 mM/min, 95.5%, and >99%, respectively, after a reaction time of around 5 h.




Similar content being viewed by others
References
Kaminskas, E., Farrell, A. T., Wang, Y. C., Sridhara, R., & Pazdur, R. (2005). Oncologist, 10, 176–182.
Romanová, D., & Novotný, L. (1996). Journal of Chromatography B, 675, 9–15.
Shafiee, M., Griffon, J. F., Gosselin, G., Cambi, A., Vincenzetti, S., Vita, A., et al. (1998). Biochemical Pharmacology, 56, 1237–1242.
Matín, D., Teijeiro, C., & Piňa, J. J. (1996). Journal of Electroanalytical Chemistry, 407, 189–194.
Beisler, J. A., Abbasi, M. M., Kelley, J. A., & Driscoll, J. S. (1977). Journal of Medicinal Chemistry, 20, 806–812.
Beisler, J. A. (1978). Journal of Medicinal Chemistry, 21, 204–208.
Ghosh, M. K., & Mitra, A. K. (1991). Pharmaceutical Research, 8, 771–775.
Siedlecki, P., Boy, R. G., Comagic, S., Schirrmacher, R., Wiessler, M., Zielenkiewicz, P., et al. (2003). Biochemical and Biophysical Research Communications, 306, 558–563.
Li, X. F., Zong, M. H., Wu, H., & Lou, W. Y. (2006). Journal of Biotechnology, 124, 552–560.
Secundo, F., & Carrea, G. (2002). Journal of Molecular Catalysis. B, Enzymatic, 19–20, 93–102.
Ferrero, M., & Gotor, V. (2000). Monatshefte für Chemie, 131, 585–616.
Morís, F., & Gotor, V. (1993). Journal of Organic Chemistry, 58, 653–660.
Mei, Y., Miller, L., Gao, W., & Gross, R. A. (2003). Biomacromolecules, 4, 70–74.
Li, X. F., Lou, W. Y., Smith, T. J., Zong, M. H., Wu, H., & Wang, J. F. (2006). Green Chemistry, 8, 538–544.
Ganske, F., & Bornscheuer, U. T. (2005). Journal of Molecular Catalysis. B, Enzymatic, 36, 40–42.
Wehtje, E., Kaur, J., Adlercreutz, P., Chand, S., & Mattiasson, B. (1997). Enzyme and Microbial Technology, 21, 502–510.
Ducret, A., Trani, M., & Lortíe, R. (1998). Enzyme and Microbial Technology, 22, 212–216.
Han, J. J., & Rhee, J. S. (1998). Enzyme and Microbial Technology, 22, 158–164.
Ma, L., Persson, M., & Adlercreutz, P. (2002). Enzyme and Microbial Technology, 31, 1024–1029.
Wang, H., Zong, M. H., Wu, H., & Lou, W. Y. (2007). Journal of Biotechnology, 129, 689–695.
Therisod, M., & Klibanov, A. M. (1986). Journal of the American Chemical Society, 108, 5638–5640.
McCabe, R. W., & Taylor, A. (2004). Enzyme and Microbial Technology, 35, 393–398.
Wang, N., Chen, Z. C., Lu, D. S., & Lin, X. F. (2005). Bioorganic & Medicinal Chemistry Letters, 15, 4064–4067.
Uppenberg, J., Öhrner, N., Norin, M., Hult, K., Kleywegt, G. J., Patkar, S., et al. (1995). Biochemistry, 34, 16838–16851.
Fan, H., Kitagawa, M., Raku, T., & Tokiwa, Y. (2004). Biotechnology Letters, 26, 1261–1264.
Wehtje, E., Costes, D., & Adlercreutz, P. (1997). Journal of Molecular Catalysis. B, Enzymatic, 3, 221–230.
Halling, P. J. (1994). Enzyme and Microbial Technology, 16, 178–206.
Bell, G., Halling, P. J., Moore, B. D., Partridge, J., & Rees, D. G. (1995). Trends in Biotechnology, 13, 468–473.
Klibanov, A. M. (1997). Trends in Biotechnology, 15, 97–101.
Li, X. F., Zong, M. H., & Yang, R. D. (2006). Journal of Molecular Catalysis. B, Enzymatic, 38, 48–53.
Degn, P., & Zimmermann, W. (2001). Biotechnology and Bioengineering, 74, 483–491.
Weber, H. K., Weber, H., & Kazlauskas, R. J. (1999). Tetrahedron: Asymmetry, 10, 2635–2638.
Morís, F., & Gotor, V. (1993). Tetrahedron, 49, 10089–10098.
Klibanov, A. M. (2001). Nature, 409, 241–246.
Acknowledgement
We acknowledge the National Natural Science Foundation of China (Grant No. 20676043), Science and Technology Project of Guangdong Province (Grant No. 2006A10602003; 2007B011000005), Science and Technology Project of Guangzhou (Grant No. 2007Z3-E4101), the Natural Science Foundation of Guangdong Province (Grant No. 05006571), the Doctoral Program of Higher Education (Grant No. 20070561080) and the Open Project Program of the State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (Grant No. N-06-06) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, XY., Zong, MH., Lou, WY. et al. Highly Efficient Regioselective Synthesis of 5′-O-lauroyl-5-azacytidine Catalyzed by Candida antarctica Lipase B. Appl Biochem Biotechnol 151, 21–28 (2008). https://doi.org/10.1007/s12010-008-8152-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8152-0