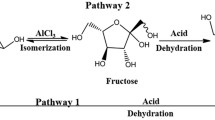
Abstract
Lanthanides were tested (Ce3+, Er3+, and Yb3+) as catalysts to produce lactic acid (LA) from the monosaccharides present in corn stover (glucose, xylose, and arabinose) resulting in ytterbium being the most active. A MW-heated system led to similar LA yield as a conventionally heated pressurized system. The maximum value of LA yield was 40% at 240 °C after 20 min of isothermal treatment regardless the starting monosaccharides, which allowed to propose a similar LA production route based on the products profile determined along time for the three monosaccharides. Temperature and time determined the product profile, observing furfural degradation at severity factors higher than 3.5, while values higher than 5.5 were needed to observe LA degradation. By increasing temperature, catalyst solubility decreased, increasing its presence in the solid residue after treatment. Xylan conversion to LA was similar as for xylose, but lower yield was obtained from microcrystalline cellulose. Corn stover presented more amorphous regions leading to higher hydrolysis yields of its cellulose fraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass is one of the most attractive options to achieve sustainable production of energy and chemicals as substitutes for petroleum products (Akram et al., 2023; Deivayanai et al., 2022; Mujtaba et al., 2023; Saravanan et al., 2023). The generation of lignocellulosic biomass by different industries is encouraging the implementation of a biorefinery approach to obtain different valuable products from a waste stream. This a sustainable approach not only to obtain value-added products, including energy, but also a variety of chemical compounds that can be obtained from biomass components. The main idea is to use the biorefinery and circular economy concept to obtain several valuable products with prospects for applications in the food, pharmaceutical, and cosmetics industries (da Silva et al., 2023). One of the most promising sources of lignocellulosic biomass is the residues generated in the agricultural industry. Different residues from agricultural industry, such as pomace, peel, husk, seed, stover, and straw have been used to obtain products, such as biomass-derived mono- and polysaccharides from which different building blocks can be obtained either by biochemical or thermochemical routes (Costa et al., 2023). Moreno-González and Ottens demonstrates that multiple high-value products can be recovered from a single agri-food side stream depending on the processing steps and the origin source (Moreno-González & Ottens, 2021). It has been estimated that the real potential of agricultural crop residues is 74.89 Mt year−1 in the European Union (Hassan et al., 2019). Lignocellulosic biomass presents not only a valuable but also variable chemical composition of mainly cellulose, hemicellulose, and lignin (Lee et al., 2021). Biomass-derived monosaccharides, hexoses and pentoses, can be used as raw materials for further conversion into a range of value-added products. Among these compounds, organic acids are considered among the main basic components derived from monosaccharide.
Lactic acid (LA) is identified as one of the top 15 platform chemicals derived from lignocellulosic biomass (Castillo Martinez et al., 2013; Dusselier et al., 2013; Xu et al., 2020). It is one of the most used organic acids, with a long history of usage in preservation of foodstuffs (Silveira et al., 2012). Furthermore, the rapid growth of the pharmaceutical industry boosts also the lactic acid market due to the growing need for prescription grade creams and medicines (Jeyavishnu et al., 2021). It is also considered an important industrial product with an expanding market due to its attractive and valuable multifunctional properties, especially in the polylactic acid (PLA) industry (Delidovich et al., 2016; Zhu et al., 2016). For all these reasons, lactic acid presents an annual growth of 16.2%. It is expected that the world lactic acid market will increase up to 1960.1 kt in 2025, while in 2016, it was only 1220.0 kt (Alves de Oliveira et al., 2018).
Currently, lactic acid is mainly produced from the fermentation of pure monosaccharides and edible crops (glucose, sucrose, starch, etc.) by using genetically modified enzymes (Abdel-Rahman et al., 2013; Liu et al., 2015; Ögmundarson et al., 2020). However, the use of edible crops as raw materials inevitably leads to competition with human food (Luterbacher et al., 2014; Mäki-Arvela et al., 2014). On the other hand, fermentation presents a limited performance in space-time and a difficult control of the reactor for the fermentation (pH and temperature) and of the microorganisms (Abdel-Rahman et al., 2013). Furthermore, lignocellulosic biomass is rarely used as raw material for the production of lactic acid by fermentation. Cellulose and hemicellulose are not directly available for bioconversion due to their interaction with lignin and the lack of hydrolytic enzymes (Ding et al., 2012; Kumar & Wyman, 2013). Fermentation of mixed monosaccharides (hexose and pentose) is also a challenge. For all these reasons, alternative ways of producing lactic acid are needed.
Recently, hydrothermal catalysis has been presented as a potential strategy for the cost-effective production of lactic acid, particularly when real lignocelluloses are used directly as feedstock (Deng et al., 2023). This technique is based on the properties of water at subcritical conditions (between 100 and 374 °C, using pressures up to 220 bar). The ionic product of water increases significantly as the temperature increases, subsequently increasing its autoionization and the concentration of acidic and basic ions in solutions and ultimately acting as a catalyst for ionic reactions, thus acting as a substitute for acids and commonly used diluted bases (Costa et al., 2023; Gomes-Dias et al., 2022). Biomass hydrolysis and fractionation in subcritical water has been extensively reviewed in the literature (Cocero et al., 2018). Recently, Costa et al. (2023) review subW as a technology to extract valuable compounds from agri-food industry by-products witing a biorefinery concept (Costa et al., 2023). The present work emphasises the use of specific catalysts to modify the reaction process in subW medium. The use of specific catalyst in hydrothermal treatments for lactic acid production has been reviewed by Li et al. (2019). The authors divided the catalytic systems into alkaline, Lewis acid, and redox systems. The use of alkaline catalysts has the disadvantage of requiring an extra acidification treatment of the alkaline lactate in which gypsum is produced as a by-product and redox systems present a complex mechanism that includes decarbonization steps, which limit the overall performance due to the release of CO2. Lewis acid catalysts appear to be the most promising catalytic system. In addition, rare earth metals, including scandium, yttrium, and lanthanides (Ln), have recently been proposed as new types of Lewis acid catalysts. Compared to typical Lewis acid catalysts, rare earth metals are cheaper, easier to recover, and are therefore considered green catalysts (Zhang et al., 2021).
Exposure of systems to elevated temperatures and long periods of operation can reduce performance and selectivity of operation. At this point, microwave irradiation can be used in the hydrothermal reaction to improve yield and selectivity under milder conditions (Liu et al., 2022; Nüchter et al., 2004). Combining subcritical with microwave can increase process efficiency and promote sustainable economy (Costa et al., 2023). Microwave heating can significantly lower the overall reaction temperature and shorten the reaction time compared to conventional heating, by directly transferring microwave energy to the internal energy of the reactants, thereby saving energy (Huang et al., 2016; Luo et al., 2017). In this way, the efficiency of the catalytic transformation of carbohydrates into lactic acid can be increased.
In this work, the feasibility of producing lactic acid by hydrothermal treatment of mono- and polysaccharides derived from lignocellulosic biomass was studied. Specifically, the study focused on corn stover as lignocellulosic biomass to be valorized. The influence of the presence of different lanthanide Lewis acid catalysts (Ce3+, Er3+, and Yb3+) on LA production was evaluated. A parametric study is carried out on the production of lactic acid, the influence of the catalyst, its concentration, and the temperature. Lactic acid production kinetics, pressure, and pressurization agent are also analyzed. The analogies and differences between conventional heating and microwave-assisted heating are studied. The reaction mixture compounds’ profile for the three main monosaccharides present in corn stover will be compared (glucose, xylose, and arabinose) and lactic acid yield will be also compared with the results obtained for the corresponding polysaccharides, pure cellulose, and xylan. The influence of the operational conditions on the degradation of the lactic acid produced is also analyzed and compared to xylose dehydration products, furfural. In addition, possible routes for lactic acid production are proposed according to the kinetic product profile from the two main monomer monosaccharides from biomass, xylose, arabinose, and glucose. Finally, the feasibility of estimating corn stover conversion to lactic acid by simple monosaccharides and polysaccharides is studied.
Experimental
Materials
The corn stover used in this work was kindly provided by Castilla y León farmers (Saldaña, Palencia). It was naturally dried by leaving it in contact with the ambient air for several days and then milled in a Retsch SM100 mill to get a final fineness of particle size of < 1 mm. The raw material was kept refrigerated at 4 °C until use.
Xylose, glucose, and xylan from corn core were purchased from TCI. Arabinose, cellulose, and lactic acid solution (40% in H2O) were purchased from Merck. Ce(NO3)3·6H2O, Er(NO3)3·5H2O, and Yb(NO3)3·5H2O were purchased from Thermo Scientific.
Equipment for Hydrothermal Treatment
Catalyzed hydrolysis was carried out in batch mode in two different reactors: a microwave (MW)-heated Teflon reactor and a conventionally heated stainless-steel (SS) reactor.
MW-Heated Teflon Reactor
A flexiWAVE MA186-002 equipment from Milestone, provided with a high-pressure Teflon reactor (up to 100 bar), was used. The system was provided with temperature control but pressure could not be set. It was just built in the reactor with increasing temperature according to the thermodynamics of the reaction mixture in a constant volume (100 mL) closed vessel. Temperature was measured with a thermocouple inserted inside of the reactor. The maximum heating power of the system was limited to 1000 W.
In a typical run, the reactor was loaded with 50 mL of reacting solution, and then, the reaction conditions in the MW chamber were set. After some heating and isothermal reaction time, the microwave chamber was cooled with the help of a built-in fan. The reactor was removed once the temperature of the chamber was below 80 °C. The reaction solution was filtered and kept under refrigeration conditions until analysis.
This reactor was used to study the influence of the type of catalyst and its concentration, as well as the influence of temperature on the reaction performance of different monosaccharides. Studies on lactic acid degradation and on the possibility of estimating the corn stover conversion from the conversion of its simple monosaccharides and polysaccharides constituents were also carried out using this reactor.
Conventionally Heated Stainless Steel Reactor
A stainless-steel (SS) reactor of 500-mL capacity from ZZKeda was also used in this work. In this case, heating was performed with a jacketed electrical heater into which the reactor fits and from which it can be easily detached for rapid cooling. Pressure inside the reactor, necessary to work with water under subcritical conditions, was achieved by adding a pressurizing agent (N2 or CO2). For CO2 pressurization, a syringe pump with pressure and volume controllers (ISCO 260 D) was used. Pressurization with N2 was achieved by connecting the reactor to a compressed N2 cylinder. A needle valve (Autoclave Engineers) followed by a cooling system allowed sampling along time. Liquid samples were taken through a sintered stainless steel microfilter with a pore size of 10 µm.
In a typical run, the reactor was loaded with 220 mL of reacting solution, and then, reaction conditions were set. Pressure was set by adding a pressurizing agent (N2 or CO2) for water to remain in its liquid state when rising temperature. After some heating time, at approximately 5 °C min−1, and certain isothermal time, along which aliquot sampling was performed at regular time intervals, the reactor was detached from the heating jacket, cooled with a fan, and depressurized when the temperature was below 80 °C. The reaction solution was filtered and kept under refrigeration conditions until analysis.
In this reactor, the effect of temperature, pressure, and pressurizing agent on the reaction kinetics was studied. The distribution of reactants and products over time was analyzed to gather information and suggest a possible operation route.
According to the literature, the effect of pressure on hydrolysis performance has been observed to be non-significant, compared with temperature and time, as long as water remains in its liquid state (Rivas-Vela et al., 2021). Therefore, the behavior of both reactors is expected to be similar, since, in both cases, reaction takes place in liquid water, either near saturation or in subcritical conditions. The MW-heated Teflon reactor has the advantage of allowing us to perform parametric screening in a simple way, as well as the recovery of almost all of the solids produced due to the absence of internal elements and the steel reactor allows to take aliquots over time, operate with more volume, and modify the pressure and the pressurizing agent.
Analytical Methods
Corn Stover Characterization
Chemical characterization of corn stover was performed in duplicate according to the standard protocols of the National Renewable Energy Laboratory (NREL Laboratory Analytical Procedures) to determine structural carbohydrates. The detailed description can be found in the NREL protocols (Sluiter et al., 2010). The extractive-free corn stover was subjected to two stages of acid hydrolysis. First, 0.3 g of dry corn stover were mixed with 3.0 mL of 72% H2SO4 (w/w) and incubated at 30 °C for 1 h. Then, the sample was diluted to 4% (w/w) H2SO4 by adding 84 mL of deionized water and autoclaved at 121 °C for 1 h. The hydrolyzate was then cooled to room temperature and vacuum filtered through a 0.7-µm pore size hydrophilic fiberglass filter (Millipore).
For the correct determination of pentoses and hexoses in the hydrolysate, sugar recovery standards (SRS) were used to account for the degradation of the monosaccharide in the sample during the dilute sulfuric acid step. SRS and corn stover hydrolysates were neutralized with CaCO3 to pH 6 and filtered through 0.2-μm syringe filters. Monosaccharides were determined by high-performance liquid chromatography (HPLC) with a Bio-Rad Aminex-HPX-87H column, a variable wavelength detector (VWD), and a refractive index detector (RID). The mobile phase used consisted of 0.005 M sulfuric acid. The temperature of the column and the RID was 40 °C. The sample volume injected was 10 μL.
Characterization of Liquid Streams from Water Hydrolysis Treatments
The determination of total organic carbon (TOC) in solution was carried out in a Shimadzu total organic carbon analyzer (TOC-V CSN). Potassium hydrogen phthalate and sodium hydrogen carbonate were used as standards.
The quantitative analysis of the reagents and products of the liquid phase was carried out by HPLC by using the same column and method described in “Corn Stover Characterization.” The amount of each reactant and product was determined using calibration curves generated with standard solutions.
The monosaccharide conversion was determined as follows:
On the other hand, the yield of each reaction product was evaluated as follows:
Characterization of Solids from Water Hydrolysis Treatments
Elemental composition (C, H, N, S, and O) of the solids was determined by an organic elemental microanalyzer equipment (Thermo Scientific Model Flash 2000).
Statistical Analysis
All values were expressed as mean ± standard deviation of at least two replicates. The significance of the differences was determined based on an analysis of the variance with the Fisher’s least significant difference (LSD) method at p-value ≤ 0.05.
Results and Discussion
Characterization of Corn Stover
Table 1 shows the weight percentages of glucans, xylans, and arabinans contained in the corn stover used in this work. Corn stover was composed of 36.5% glucans, 20.2% xylans, and 3.6% arabinans in a dry basis. Consequently, 60.3% of the corn stover are polysaccharides, which could be potentially converted into chemical platforms such as lactic acid.
Parametric Study of Lactic Acid Production from Simple Monosaccharides
In this section, the study of the influence of several process parameter on the production of lactic acid from glucose and xylose is presented. These monosaccharides were the most abundant simple monosaccharides in corn stover (Table 1). The process parameters studied were the following: the presence of catalysts, such as the Lanthanides Lewis acid catalysts Ce3+, Er3+, or Yb3+, its concentration, and the reaction temperature. The MW-heated Teflon reactor was used for this parametric study.
Catalyst Influence
To study the influence of the type of catalyst on the conversion and reaction yield, a reaction temperature of 200 °C (5 min heating, 20 min isothermal) was selected. Monosaccharide concentration was fixed at 0.05 M. The individual catalytic hydrolysis was studied by using Ce(NO3)3, Er(NO3)3, and Yb(NO3)3 as potential catalysts with a 0.005 M catalyst concentrations. Zhang et al. (2021) studied the effect of counterions and observed that YbCl3, Yb(NO3)3, and Yb(OTf)3 catalysts showed similar activity for the conversion of glucose to lactic acid. However, Yb2(SO4)3 catalyst provided a slightly lower yield and Yb(CH3COO)3 and Yb2(C2H2O4)3 catalysts a fairly low yield. The authors suggested that the activity of Yb(III) salts with different anions depends on their solubility in water. YbCl3, Yb(NO3)3, and Yb(OTf)3 dissolve completely in water, thus providing higher activity. Therefore, from this point on, the catalysts used in this work will be referred to as follows: Ce3+, Er3+, and Yb3+. Figure 1 shows the yields of the different reaction products for the experiments catalyzed by Ce3+, Er3+, and Yb3+, starting from the reference simple monosaccharides. The results obtained without catalyst are also included for comparison. Table 2 collects the conversion values of the starting monosaccharides for the different combinations analyzed.
Yields of the different reaction products for the combinations of catalysts (Ce3+, Er3+, Yb3+, and without catalyst) and monosaccharides (xylose and glucose). Experiments carried out in a MW heated Teflon reactor at 200 °C (5 min heating, 20 min isothermal) and 0.05 M monosaccharide and 0.005 M catalyst concentrations. Values with different letters for each compound are significantly different when applying the Fisher’s least significant differences (LSD) method at p-value ≤ 0.05
In the operation without catalyst, monosaccharide conversion was limited; only 35.7% of xylose and 30% of glucose (Table 2) were converted. Furthermore, lactic acid was not detected for either of the two starting simple monosaccharides (Fig. 1). Xylose was mainly converted to furfural (Fig. 1a) and glucose to HMF (Fig. 1b), which are the dehydration products of monosaccharides (Deng et al., 2018; Kiatphuengporn et al., 2020; Wang et al., 2013).
With the incorporation of a catalyst, the conversion of the starting monosaccharide increased remarkably. Most combinations reached almost total conversion (> 99.4%), while the experiment with glucose and Ce3+ reached 95.8%. It is shown that the presence of Lewis acid catalysts markedly improved monosaccharide conversion. Furthermore, with the incorporation of the catalyst, the distribution of products varied, and lactic acid became the major product. The suitability of the catalysts will be defined by the maximization of lactic acid production and the limitation of furfural formation. It was confirmed that the selected catalysts were active and selective in the production of lactic acid. Comparing the catalysts with each other, Ce3+ presented the lowest performance. Only 17.5 and 13.1% of lactic acid were obtained for xylose and glucose, respectively. Also, note that the conversion of glucose in the presence of Ce3+ presented the lowest value (95.8%). Consequently, Ce3+ was not selected as a suitable catalyst for lactic acid production.
The LA yield increased up to 34.2% (xylose, Fig. 1a) and 26.4% (glucose, Fig. 1b) with the presence of Er3+, and ytterbium (Yb3+) provided the highest lactic acid yield for the two reference monosaccharides studied (38.0% xylose and 30.9% glucose); therefore, Yb3+ was selected as the most suitable catalyst for further studies. In addition, the production of lactic acid was about 4.5 times higher compared to the monosaccharide’s dehydration products (furfural and HMF). Zhang et al. (2021) concluded that the activity of lanthanide (III) ions increases with atomic number, as well as with decreasing ionic radius. These conclusions agree with the present work since the atomic number is 58, 68, and 70 for Ce, Er, and Yb, respectively, and the ionic radius (Van der Waals radius) is 248, 232, and 228 pm, respectively. Zhang et al. (2021) proposed that the oxidation potential, increasing with atomic number, is what could facilitate the disproportionation reaction in the conversion of glucose through an intramolecular H shift.
Influence of Catalyst Concentration
Figure 2 shows the evolution of the yields of the different reaction products, for the experiments carried out at different concentrations of catalyst and for both monosaccharides. Yb3+ was selected as catalyst given the best results obtained (Fig. 1) regarding Ce3+ and Er3+. The experiments were carried out in a MW-heated Teflon reactor at 240 °C (10 min heating, 10 min isothermal). On this occasion, the isothermal time was reduced to 10 min to have more resolution in the influence of catalyst concentration. The monosaccharide concentration was set to 0.05 M. Table 3 collects the conversion values of the starting monosaccharides for the different combinations analyzed.
Yields to the different compounds for the combinations of catalyst (Yb3+) concentration and monosaccharides (xylose and glucose). Experiments carried out in a MW heated Teflon reactor at 240 °C (10 min heating, 20 min isothermal) and 0.05 M monosaccharide concentration. Values with different letters for each compound are significantly different when applying the Fisher’s least significant differences (LSD) method at p-value ≤ 0.05
The production of lactic acid presented a volcano trend for both monosaccharides (Fig. 2) and the conversion of the starting monosaccharides was almost complete (Table 3). The presence of 0.0005 mol L−1 of catalyst provided a monosaccharide conversion greater than 99.4% and produced a lactic acid yield greater than 30% for both monosaccharides. On the other hand, the yields of the dehydration products of pentoses and hexoses were limited to 14.3% (furfural, Fig. 2a) and 6.9% (HMF, Fig. 2b), respectively. This fact reveals the high activity of the selected catalyst (Yb3+) which, with a very low concentration, lead to high lactic acid production yields. Doubling the catalyst concentration (0.001 mol L−1) increased the lactic acid yield up to 36% and limited the dehydration products to 10.5 and 3.9% for xylose and glucose, respectively.
The catalyst concentration of 0.005 mol L−1 provided almost a 40% yield of lactic acid, corresponding to the maximum production. Further increase in concentration to 0.01 mol L−1 limited the yield to lactic acid. Ma et al. ( 2023) also observed a positive effect on lactic acid yield by increasing the ErCl3 concentration from 0.0033 to 0.0067 mol L−1. However, higher catalyst concentrations (0.01–0.0133 mol L−1) presented lower yield. The authors suggested that it could be due to side reactions or additional reactions of lactic acid to other by-products.
In addition, in the case of xylose (Fig. 2a), increasing catalyst concentration to 0.01 mol L−1 led to lower furfural concentration; however, for glucose, the HMF yield increased with increasing catalyst concentration. In parallel, levulinic performance was also diminished in glucose hydrolysis.
To check for any additional side reaction, the concentration of solids in the final liquid and the percentage ratio with respect the initial solid concentration (Solidf/Solidi) is also reported (Table 3). As the catalyst concentration increased, the solid concentration also increased, suggesting that the decrease in lactic acid yield and other secondary reaction products, such as furfural, may be due to a higher production of solids.
Influence of the Operating Temperature
The effect of the operating temperature was studied in the MW-heated Teflon reactor at 200–260 °C (10 min heating, 10 min isothermal). The concentration of monosaccharides (xylose, arabinose, and glucose) was set to 0.05 M and to 0.005 M for catalyst (Yb3+). Figure 3 shows the yields of the different reaction products at the different operating temperatures for all the monosaccharides. In parallel, the conversions of the monosaccharides, the concentration of solids in the final liquid, and the percentage ratio with respect to the initial solid concentration (Solidf/Solidi) are collected in Table 4. Almost total conversion values (> 98.3%) were obtained, which increased as the operating temperature increased; in fact, at 260 °C, the conversion was 100% for the 3 reference monosaccharides. In the operation with xylose (Fig. 3a), there was an increase in the yield of the lactic acid produced with the increase in temperature; however, at 260 °C the yield was slightly reduced. Therefore, the temperature of 240 °C was considered as optimal for the conversion of xylose to lactic acid.
Influence of temperature on yields to the different compounds for xylose, arabinose, and glucose. Experiments carried out in a MW-heated Teflon reactor at 200–260 °C (10 min heating, 10 min isothermal) and 0.05 M monosaccharide and 0.005 M catalyst (Yb3+) concentrations. Values with different letters for each compound are significantly different when applying the Fisher’s least significant differences (LSD) method at p-value ≤ 0.05
The results obtained for arabinose are collected in Fig. 3b. The observed trend is very similar to that of xylose (Fig. 3a). This was expected since both monosaccharides are pentoses with the same empirical formula. Therefore, the temperature of 240 °C was also selected as optimal for the conversion of arabinose to lactic acid. Finally, Fig. 3c shows the results corresponding to glucose. The lactic acid yield presented an upward trend with temperature and similar yields were obtained at 240 and 260 °C; thus, 240 °C was considered as optimal as well. The similar optimum temperature determined for the three main monosaccharides present in corn stover is a key finding since the main monomers present in the lignocellulosic biomass would produce lactic acid at the same optimum working temperature.
Regarding the evolution of the dehydration products, furfural (Fig. 3a, b) and HMF (Fig. 3c), it is observed how they present a downward trend with temperature. Therefore, the increase in temperature, in addition to increasing the lactic acid yield, improved its yield and selectivity by limiting dehydration reactions. On the other hand, the increase in temperature also led to an increase in the concentration of solids, as well as a darker solution, which is assigned to a higher concentration of solids favored by high temperatures. Deng et al. (2018), in the conversion of cellulose in the presence of homogeneous acid catalysts (Al(III)–Sn(II)), observed that the reaction solution turned dark brown with the increase in the operating temperature. The authors assigned this phenomenon to increased formation of unwanted humins.
Kinetics of Production of Lactic Acid from Simple Monosaccharides
Once the type of catalyst and its concentration was selected, the lactic acid production kinetics in subW, from xylose, was determined at different temperatures and pressures and by using different pressurization agents.
Effect of Reaction Temperature on LA Production Kinetics
The study of the effect of the operating temperature was extended in a batch SS reactor by determining the lactic acid production kinetics by withdrawing samples at regular time intervals. The concentrations were fixed to 0.005 M for the catalyst (Yb3+) and to 0.05 M for xylose. Figure 4 shows the lactic acid yields along time for experiments carried out in the temperature range from 180 to 240 °C. It must be highlighted that zero time corresponds to the instant that the desired temperature is reached in the reactor.
Influence of temperature on lactic acid yield along operation time. Experiments carried out in a SS reactor at 180–240 °C, 50 bar (pressurized with N2), and 0.05 M xylose and 0.005 M catalyst (Yb3+) concentrations. Zero time corresponds to the instant that the desired temperature was reached in the reactor
The operation at 180 °C presented a maximum yield of 31.5% at 30 min of isothermal treatment, and then, the lactic acid produced continued stable without degrading during the 250 min of isothermal operation. The increase in the operating temperature up to 200 °C increased the yield to 34.6% also at 30 min of isothermal treatment and also remained stable along the studied operating time. A further temperature increase, up to 220 °C, continued to increase the maximum performance obtained. On this occasion, a yield of 37.0% was achieved at a shorter operating time (20 min). It can be observed that a higher operating temperature favors a higher reaction rate; however, at 220 °C, degradation of the lactic acid produced was significant. The yield was reduced to 31.6% after 250 min of operation. Finally, the last temperature increase (240 °C) again increased the yield (39.5% at 20 min). However, at such a high operating temperature, a faster degradation of the lactic acid produced was observed. As a result, after 250 min of operation, the yield was reduced to 29.8%, being the lowest value among the four temperatures studied.
In this context, the importance of the joint selection of the operating temperature and the duration of the isothermal time is highlighted. The choice of an overestimated isothermal time will limit the yield due to the degradation of the lactic acid produced. On the other hand, the choice of a low operating temperature or a short isothermal time will limit the performance that can be achieved. Cao et al. (2017), in the conversion of glucose catalyzed by Nb2O5, observed an increase in the lactic acid yield from 19.5 to 38.6% when heated from 170 to 250 °C, for a reaction time of 4 h. However, it was reduced down to 34.5% at 270 °C. The authors explained this behavior considering the lactic acid degradation under the hydrothermal conditions used. This result agrees with the results obtained in this work. In Fig. 4, it can be clearly seen how a higher operating temperature leads to a greater degradation of the lactic acid produced. Furthermore, based on the results observed in Fig. 4, it appears that an increase in operating temperature would continue to increase performance, although higher temperatures could not be essayed due to limited maximum specifications of the SS reactor. However, in the previous section, it was observed that the maximum yield was obtained at 240 °C from xylose an arabinose and the yields at 240 and 260 °C were the same for glucose. Therefore, considering previous results, 240 °C was confirmed as optimal operation temperature.
The combined effect of temperature and reaction time during hydrothermal processes was evaluated by the severity factor parameter according to Eq. (3) (Alonso-Riaño et al., 2023; Ruiz et al., 2021; Wang et al., 2016):
where R0 is the severity factor, t is the treatment time (min), and T is the operating temperature (°C). The reference temperature is fixed at 100 °C because under this temperature no significant solubilization nor depolymerization of the hemicellulose/cellulose take place, and the value 14.75 is a typical activation energy for glycosidic bond cleavage of carbohydrates under hydrothermal treatment assuming conversion is first order.
Figure 5a shows the production of lactic acid as a function of the logarithm of the severity factor. For the operation at 180 and 200 °C, a decrease in lactic acid production was not observed, regardless of the logarithm of the severity factor. Log(R0) yielded values lower than 5.5 in the time range covered in this work at the lowest temperature essayed, 180 and 200 °C. These Log(R0) values were not high enough to lead to lactic acid degradation. However, from Log(R0) values of 5.5, a decrease in lactic acid production can be observed. This behavior highlights the importance of the joint selection of temperature and operating time. In the case of the operation at 200 °C, a time of 360 min would be necessary to reach a Log(R0) value of 5.5, while in the operation at 180 °C, the time would increase up to 1400 min.
To compare the behavior under subcritical water conditions of the other major reaction products from xylose, Fig. 5b shows the production of furfural as a function of the logarithm of the severity factor. In the previous section, it has been observed how the increase in the operating temperature decreases the furfural yield (Fig. 3). Figure 5b shows the same trend. In addition, a downward trend was seen for all temperatures with the increase of Log(R0), regardless its value. Therefore, it is shown that the lactic acid produced from xylose (desired product) was much more stable than its dehydration product (furfural).
Finally, the elemental composition of the solids generated during the experiments carried out at different temperatures was analyzed and included in Table 5. The H/C and O/C ratios are also determined, and Fig. 6 shows the Van Krevelen diagram for the xylose and the solids obtained at the different operating temperatures. It is observed how after subcritical water chemo-catalysis of xylose, both ratios were reduced and very close ratios were obtained. In the case of the H/C ratio, values of 0.65–0.70 were obtained and in the case of the O/C ratio values of 0.42–0.47. Slope values of 2.27–2.55 were obtained in the Van Krevelen diagram, suggesting that most solids could come from xylose dehydration products, namely, furfural (Licursi et al., 2015; Pérez et al., 2018).
Regarding N content of the solid fraction, it can be observed in Table 5 that as the operating temperature increased, the amount of nitrogen increased from 0.3% for the operation at 180 °C to 1.3% for the operation at 240 °C. Since the starting xylose does not contain nitrogen in its composition, it is suggested that the nitrogen comes from the catalyst (Yb(NO3)3·5H2O) added to the medium. On the other hand, the increase in the concentration of N is assigned to the fact that, as the operating temperature increases, the dielectric constant of water in subcritical water state decreases (Akiya & Savage, 2002; Cocero et al., 2018), which limits the solubility of the catalyst in the reaction medium leading to a greater amount of precipitate.
Influence of Pressure and Pressurization Agent on LA Production Kinetics
The effect of pressure and pressurization agent was studied in a batch SS reactor that allows aliquot sampling with operation time. Figure 7 shows the lactic acid yields of the different combinations of pressure (50 and 100 bar) and pressurization agent (N2 or CO2). The experiments were carried out with xylose as the reference monosaccharide and Yb3+ was used as a catalyst. The concentrations were fixed to 0.005 M for the catalyst (Yb3+) and to 0.05 M for xylose. Despite the fact that 240 °C has been selected as the optimum operating temperature, the experiments were carried out at 200 °C to clearly observe degradation of lactic acid due to pressure and/or pressurization agent. Zero time corresponds to the instant that the desired temperature was reached in the reactor. Figure 7 presents the same trend for the different combinations of operating pressure and pressurization agent. In general, there was a sharp increase in lactic acid yield up to 20–30 min of isothermal treatment and afterwards, it remained constant during the 250 min of operation. Therefore, it can be concluded that the pressure increase up to 100 bar or the use of CO2 as pressurization agent do not influence the degradation of lactic acid and this only depends on the operating temperature.
Influence of pressure (50–100 bar) and pressurization agent (N2/CO2) on lactic acid yield along operation time. Experiments carried out in a SS at 200 °C and 0.05 M xylose and 0.005 M catalyst (Yb3+) concentrations. Zero time corresponds to the instant that the desired temperature is reached in the reactor
The increase in pressure from 50 to 100 bar has a slight positive effect for both pressurization agents. The yield at 30 min of isothermal treatment increases from 34.6% at 50 bar to 35.3% at 100 bar when using N2. Therefore, it is concluded that pressure should be simply enough to keep water in its liquid state when dealing with an inert gas such as N2. Rivas-Vela et al. (2021) observed how insignificant the effect of pressure was on the hydrolysis yield compared to temperature and time, as long as the water remains in a liquid state. Regarding the influence of the nature of the pressurization agent, the use of CO2 in hydrothermal processes promotes an enhanced acid reactive environment, since CO2 is partially dissolved in water (Benito-Román et al., 2019; Melgosa et al., 2020, 2021). Pressurizing with CO2 instead of with an inert gas (N2) improved performance from 34.6 to 36.7% when working at 50 bar. Finally, the highest yield (37.3%) was obtained in the experiment carried out at 100 bar and pressurized with CO2, suggesting a positive effect of pressure when working with CO2. It is expected a greater effect of the presence of CO2 and operating pressure when directly hydrolysing lignocellulosic biomass instead of simple monosaccharides, improving the hydrolysis of the biomass into its different components.
Comparison Between MW-Heated Teflon and Steel Reactor Performance
In this work, two types of reactor have been used: a microwave-heated Teflon reactor that operated at saturation pressure, considering gas generation in the MW treatment, and a SS reactor pressurized with a gas, at a pressure high enough to keep water in its liquid state. In this section, the results obtained for similar operating conditions are compared. Xylose was selected as starting monosaccharide and Yb3+ as catalyst with concentrations of 0.05 M and 0.005 M, respectively. The temperature was set at 240 °C and the isothermal time was set at 10 min. Figure 8 shows the production of the different compounds in both reactors at the previously mentioned operating conditions. For all the compounds, very close results can be observed, so it can be concluded that the results obtained by both heating type and operating pressure are of the same order.
Yields of the different compounds for MW-heated Teflon and SS reactors. MW-heated Teflon reactor conditions: 240 °C (10 min heating, 10 min isothermal) and 0.05 M xylose and 0.005 M catalyst (Yb3+) concentrations. SS reactor conditions: 240 °C, 50 bar (pressurized with N2), 10 min isothermal time, and 0.05M xylose and 0.005M catalyst (Yb3+) concentrations
Lactic Acid Degradation Study
In the previous sections, the influence of temperature, pressure, and pressurization agent on lactic acid yield over time has been studied for the hydrolysis of monosaccharides. It has been observed that an increasing working pressure and the pressurization agent did not lead to a faster degradation of the lactic acid produced at 200 °C; however, higher operating temperatures did as it was described in “Effect of Reaction Temperature on LA Production Kinetics.” Therefore, a deeper study of the degradation of lactic acid with temperature was carried out. To do this, a lactic acid solution was prepared with a concentration of 3.1 g L−1 (similar to the concentrations obtained from pure monosaccharides) and its degradation behaviour with temperature was analyzed. The results have been collected in Fig. 9. The degradation study was carried out in the MW-heated Teflon reactor. Yb3+ (0.005M) was also introduced to emulate the state of the system during the experiments.
It can be observed that the lactic acid concentrations for all the degradation experiments carried out were below the initial concentration. Therefore, it can be confirmed that, in the studied temperature range (200–260 °C), lactic acid suffers degradation. With the increase in temperature, the degradation is more accentuated, as it was also observed in Fig. 4. The concentration of lactic acid after the experiment at 260 °C was reduced to 1.9 g L−1; therefore, it degrades a 39%. A detailed observation of the chromatograms allowed to detect the formation of acetic acid. Consequently, part of the lactic acid was degraded to acetic acid. Figure 9 shows how the concentration of acetic acid increases temperature, while that of lactic acid decreases.
Finally, the influence of time on lactic acid degradation was also analysed, by performing an additional experiment at 260 °C for 20 min. The results are represented also in Fig. 9. The acetic acid production was not affected by the operating time; however, it can be observed that the longer the isothermal time at 260 °C, the larger the lactic acid degradation. Specifically, LA was reduced by 45% (1.7 g L−1). These results agree with those observed in Fig. 4, in which it can be seen that the lactic acid yield was reduced over the course of the operation time when working at high temperatures.
Possible Routes of Lactic Acid Formation Depending on the Starting Monosaccharide
In this section, the distribution of products generated in the catalytic process to obtain lactic acid by using Yb3+ as catalyst at 240 °C is presented for the three main monosaccharides found in different lignocellulosic biomasses such as corn stover (xylose, arabinose and glucose). The experiments were carried out in the batch SS reactor that allows withdrawing samples along reaction time. While heating the reactor until the isothermal temperature was reached, samples were withdrawn at 50, 100, 150, 200, and 240 °C, which correspond approximately to 10, 17, 24, 34, and 53 min. From that point, samples were taken during the isothermal treatment at specific operating times. Results are presented in Fig. 10.
It can be observed that the monosaccharide concentration barely varies until 150 °C were reached (≈24 min) and from that point it drops sharply. However, the lactic acid concentration does not increase noticeably until 200 °C were reached (≈34 min). This trend was observed for the three monosaccharides studied; however, different degradation compounds were detected for each monosaccharide.
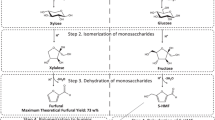
In the experiment with xylose (Fig. 10a), with the decrease in its concentration, the increase in the concentration of xylulose can be observed. With arabinose (Fig. 10b), ribulose was detected, while with glucose (Fig. 10c) fructose was the main reaction product. That is, the isomerization of aldoses to the corresponding ketoses was observed. In parallel, retroaldol condensation products, such as dihydroxyacetone and glyceraldehyde, and their dehydration compounds like pyruvaldehyde and glycoaldehyde were also detected for the three monosaccharides. These compounds were only detected during the heating step, and none of them were detected during the isothermal step at 240 °C.
The detection of these compounds is consistent with the mechanisms proposed in the literature for lactic acid production from monosaccharides. Li et al. (2019), in their review of the catalytic conversion of cellulose-derived monosaccharides to lactic acid, concluded that the conversion of soluble monosaccharides involves three key steps. First, the isomerization of monosaccharides occurs. For example, in the case of glucose, it is isomerized to fructose. Subsequently, retroaldol reactions of fructose to trioses occur, in which dihydroxyacetone and glyceraldehyde are produced. Finally, the trioses are converted to lactic acid through several reactions in tandem. In this tandem of reactions, the dehydration of glyceraldehyde to pyruvaldehyde is included.
Density functional theoretical (DFT) studies revealed that the retroaldol fragmentation of fructose is the step that required the most energy, where the activation energy barrier is 32.8 kcal mol−1 (Wang et al., 2013). In contrast, the Gibbs energy for the dehydration of fructose to HMF is relatively low (29.1 kcal mol−1), suggesting that HMF formation is more favorable in the absence of any Lewis catalyst. These results are in agreement with those shown in Fig. 1b. In the experiment without catalyst, lactic acid was not detected and HMF was the main product. However, with the presence of a Lewis acid catalyst, lactic acid was the major product. Wang et al. (2013) observed that the energy barrier for the retroaldol was significantly reduced to 22.4 kcal mol−1 with the addition of a catalyst (Pb(II)), which guided the reaction towards the selective formation of lactic acid.
Based on the literature and the time evolution of compounds observed, it is concluded that the route of formation of lactic acid from xylose, arabinose, and glucose begins with the isomerization of aldose into ketose. Next, the formation of trioses (dihydroxyacetone and glyceraldehyde) from hexoses and trioses and glycoaldehyde from pentoses takes place, which are further dehydrated to pyruvaldehyde that yields lactic acid. The proposed route of lactic acid formation is depicted in Scheme 1.
For a better comparison, Fig. 11 shows the evolution of the lactic acid yield for the three monosaccharides, once the isothermal temperature of 240 °C was reached. Glucose (C6), xylose, and arabinose (C5) present a very similar performance. Firstly, the yield increases up to 20 min of isothermal treatment and from that point, it decreases due to the degradation of the lactic acid produced. Given the similar results, it can be concluded that lactic acid degradation is independent of the starting monosaccharide. Table 6 shows the ratio between the total carbon of the final monosaccharide solution and the total carbon of the initial solution (TCf/TCi). Very close values of the ratio were obtained (0.74–0.78) for the three monosaccharides. Consequently, approximately 75% of the initial carbon remains in the liquid phase. The remaining 25% is supposed to be transformed into solid and gaseous products.
Lactic acid yields with the operation time for xylose, arabinose and glucose. Experiments carried out in a steel reactor at 240 °C, 50 bar (pressurized with N2), and 0.05M monosaccharide and 0.005M catalyst (Yb3+) concentrations. Zero time corresponds to the instant that the desired temperature is reached in the reactor
The similarity described in Fig. 11 in the results between xylose and arabinose was expected since both are C5 monosaccharides. However, glucose is a C6 monosaccharide, which could produce two molecules of lactic acid for each glucose molecule. Regarding this point, it must be highlighted that, in the literature, there are several ways to calculate the lactic acid yield. Some authors relate the moles of carbon in lactic acid to the moles of carbon contained in the starting monosaccharides (Cao et al., 2017; Ma et al., 2023). This calculation has been selected in this work to determine the lactic acid yield (Eq. 2). The yield for the other compounds present in the liquid phase was determined in the same way, considering the number of carbon moles present in each compound. Other authors, however, relate the moles of lactic acid produced with the initial moles of monosaccharide (Eq. 4) (Kiatphuengporn et al., 2020). Finally, the yield can also be determined taking into account that one lactic acid molecule can be produced from a pentose and two from a hexose (Eq. 5) (Xu et al., 2020; Ye et al., 2021).
Table 6 lists the lactic acid yields determined in the different ways described in the literature (Eqs. 2, 4, and 5) for an isothermal reaction time of 20 min. The conversion of the three reference monosaccharides, which was complete for the three of them, is also included in Table 6. For pentoses (xylose and arabinose) the Ymolar is identical to Yest. This result is due to the fact that only one molecule of lactic acid can be produced from one molecule of monosaccharide. However, for glucose (hexose), the Ycarbon is identical to Yest, since the 6 carbon atoms of the glucose molecule would produce 2 molecules of lactic acid (triose). Considering the yield values (Table 6), it is suggested that regardless the starting monosaccharide is a hexose or a pentose, only one lactic acid molecule will be produced for each monosaccharide molecule. These results are in agreement with the evolution of the products observed in Fig. 10, since for all the monosaccharides (pentoses and hexoses) the formation of glycoaldehyde (diose) was observed. Future experiments will be performed to further investigate this suggestion.
Estimation of Corn Stover Conversion of Simple Monosaccharides and Polysaccharides
The corn stover used in this work is composed of 36.5% glucans, 20.2% xylans, and 3.6% arabinans (Table 1). This section analyses the possibility of estimating the lactic acid production considering its composition on simple monosaccharides and/or its polysaccharide composition. The study was performed in the MW-heated Teflon reactor. Figure 12 shows the yields of different mono- and polysaccharide-derived compounds present in the corn stover, specifically the four following substrates: a mixture of simple monosaccharides (61% glucose, 33% xylose, and 6% arabinose), pure xylan, pure cellulose, and a mixture of the both polysaccharides (61% cellulose and 39% xylan). In addition, one experiment carried out with the selected lignocellulosic biomass, corn stover, is also included. A reaction temperature of 240 °C (10 min heating, 10 min isothermal) was selected. The concentrations were fixed to 0.005 M for the catalyst (Yb3+) and to 0.05 M for total monosaccharide concentration in base of monomers.
Yields of the different compounds for the corn stover and the four following substrates: a mixture of simple monosaccharides (61% glucose, 33% xylose, and 6% arabinose), commercial polysaccharides (61% cellulose and 39% xylan), xylan (100%), and cellulose (100%). Experiments carried out in a Teflon reactor heated by MW at 240 °C (10 min heating, 10 min isothermal), 0.05 M monosaccharide, and 0.005 M catalyst (Yb3+) concentrations
From the mixture of simple monosaccharides (61% glucose, 33% xylose, and 6% arabinose), a lactic acid yield of 37.5% was obtained, similar to the yield value for individual monosaccharides. However, from the commercial polysaccharides (61% cellulose and 39% xylan), the yield was reduced to 19.4%. Since difficulty was observed in dissolving the selected microcrystalline cellulose, additional experiments were performed, in which only xylan or cellulose were used as substrates (maintaining the same monosaccharide concentration, 0.05 M). From xylan, a similar yield (37.6%) to that of simple monosaccharides was obtained, however, from cellulose only a 3.1% yield was obtained, since it was not possible to dissolve it under the conditions used in this work. Therefore, it is concluded that the conversion of corn stover and the lactic acid yield cannot be estimated from its polysaccharide’s composition due to the impossibility of dissolving cellulose under the working conditions.
Finally, the experiment carried out directly from corn stover provided a yield of 30.3%, slightly lower than for the monosaccharide mixture. These results show the difficulty of hydrolyzing all the polysaccharides into its monosaccharide components. Therefore, it is concluded that, if the composition of a lignocellulosic biomass is known, the lactic acid production that will be obtained from its simple monosaccharides can be estimated.
Conclusions
Selective conversion of biomass-derived mono- and polysaccharides to lactic acid was achieved by using lanthanide Lewis acid catalyst in subcritical water as reaction medium. Similar results were obtained in terms of lactic acid yield by using a MW-heated Teflon reactor system compared to a conventional-heating pressurized stainless steel reactor system. Therefore, a MW-heated system helped to quickly screen the effect of the main process parameters including temperature, type of catalyst, and catalyst concentration.
On the other hand, in the external gas pressurized system, slightly better results, in terms of reaction rate and maximum conversion yield, were obtained when using CO2 as pressurization agent compared to N2. The maximum value of lactic acid yield was 40%. It was obtained at 240 °C after 20 min of isothermal treatment, by using a Yb3+ concentration of 0.005 M, regardless the starting monosaccharides (glucose, xylose, and arabinose). This finding is very important since maximum lactic acid yield could be obtained in one-step process considering a complex biomass such as corn stover. A similar lactic acid production route was proposed for all the monosaccharides involving isomerization of aldose to ketose followed by the formation of trioses, which are further dehydrated to pyruvaldehyde and finally to lactic acid.
Temperature was a key parameter not only affecting the reaction rate. The combined effect of temperature and time, severity factor, determined the distribution product profile since different behaviors was observed for lactic acid and furfural, being furfural more easily degraded. But also, a temperature increase led to a decrease in catalyst solubility in subcritical water increasing its concentration in the solid residue formed during treatment.
Xylan led to similar lactic acid yield compared to monosaccharides, but microcrystalline cellulose was not easily hydrolyzed in subcritical water, even in the presence of catalyst, and very much lower yields were obtained. Corn stover would present more amorphous regions leading to higher yields of the cellulose fraction compared to the commercial microcrystalline cellulose.
Data Availability
Research data associated with the article is available upon request by email to the corresponding author.
References
Abdel-Rahman, M. A., Tashiro, Y., & Sonomoto, K. (2013). Recent advances in lactic acid production by microbial fermentation processes. Biotechnology Advances, 31(6), 877–902. https://doi.org/10.1016/j.biotechadv.2013.04.002
Akiya, N., & Savage, P. E. (2002). Roles of water for chemical reactions in high-temperature water. Chemical Reviews, 102(8), 2725–2750. https://doi.org/10.1021/cr000668w
Akram, H. A., Imran, M., Javaid, A., Latif, S., Rizvi, N. B., Jesionowski, T., & Bilal, M. (2023). Pretreatment and catalytic conversion of lignocellulosic and algal biomass into biofuels by metal organic frameworks. Molecular Catalysis, 539, 112893. https://doi.org/10.1016/j.mcat.2022.112893
Alonso-Riaño, P., Ramos, C., Trigueros, E., Beltrán, S., & Sanz, M. T. (2023). Study of subcritical water scale-up from laboratory to pilot system for brewer’s spent grain valorization. Industrial Crops and Products, 191, 115927. https://doi.org/10.1016/j.indcrop.2022.115927
Alves de Oliveira, R., Komesu, A., Vaz Rossell, C. E., & Maciel Filho, R. (2018). Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochemical Engineering Journal, 133, 219–239. https://doi.org/10.1016/j.bej.2018.03.003
Benito-Román, O., Varona, S., Sanz, M. T., & Beltrán, S. (2019). Valorization of rice bran: Modified supercritical CO2 extraction of bioactive compounds. Journal of Industrial and Engineering Chemistry, 80, 273–282. https://doi.org/10.1016/j.jiec.2019.08.005
Cao, D., Cai, W., Tao, W., Zhang, S., Wang, D., & Huang, D. (2017). Lactic acid production from glucose over a novel Nb2O5 nanorod catalyst. Catalysis Letters, 147(4), 926–933. https://doi.org/10.1007/s10562-017-1988-6
Castillo Martinez, F. A., Balciunas, E. M., Salgado, J. M., Domínguez González, J. M., Converti, A., Oliveira, R. P., & de Souza. (2013). Lactic acid properties, applications and production: A review. Trends in Food Science & Technology, 30(1), 70–83. https://doi.org/10.1016/j.tifs.2012.11.007
Cocero, M. J., Cabeza, Á., Abad, N., Adamovic, T., Vaquerizo, L., Martínez, C. M., & Pazo-Cepeda, M. (2018). Understanding biomass fractionation in subcritical & supercritical water. Journal of Supercritical Fluids, 133, 550–565. https://doi.org/10.1016/j.supflu.2017.08.012
Costa, J. M., Strieder, M. M., Saldaña, M. D. A., Rostagno, M. A., & Forster-Carneiro, T. (2023). Recent advances in the processing of agri-food by-products by subcritical water. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-023-03071-8
da Silva, J., de Brito, E. S., & Ferreira, S. R. S. (2023). Biorefinery of cashew by-products: Recovery of value-added compounds. Food and Bioprocess Technology, 16(5), 944–960. https://doi.org/10.1007/s11947-022-02916-y
Deivayanai, V. C., Yaashikaa, P. R., Senthil Kumar, P., & Rangasamy, G. (2022). A comprehensive review on the biological conversion of lignocellulosic biomass into hydrogen: Pretreatment strategy, technology advances and perspectives. Bioresource Technology, 365, 128166. https://doi.org/10.1016/j.biortech.2022.128166
Delidovich, I., Hausoul, P. J. C., Deng, L., Pfützenreuter, R., Rose, M., & Palkovits, R. (2016). Alternative monomers based on lignocellulose and their use for polymer production. Chemical Reviews, 116(3), 1540–1599. https://doi.org/10.1021/acs.chemrev.5b00354
Deng, W., Feng, Y., Fu, J., Guo, H., Guo, Y., Han, B., & Zhou, H. (2023). Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy & Environment, 8(1), 10–114. https://doi.org/10.1016/j.gee.2022.07.003
Deng, W., Wang, P., Wang, B., Wang, Y., Yan, L., Li, Y., & Wang, Y. (2018). Transformation of cellulose and related carbohydrates into lactic acid with bifunctional al(iii)–Sn(ii) catalysts. Green Chemistry, 20(3), 735–744. https://doi.org/10.1039/C7GC02975F
Ding, S., Liu, Y., Zeng, Y., Himmel, M. E., Baker, J. O., & Bayer, E. A. (2012). How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science, 338(6110), 1055–1060. https://doi.org/10.1126/science.1227491
Dusselier, M., Van Wouwe, P., Dewaele, A., Makshina, E., & Sels, B. F. (2013). Lactic acid as a platform chemical in the biobased economy: The role of chemocatalysis. Energy & Environmental Science, 6(5), 1415–1442. https://doi.org/10.1039/C3EE00069A
Gomes-Dias, J., Teixeira, J. A., & Rocha, C. M. R. (2022). Recent advances in the valorization of algae polysaccharides for food and nutraceutical applications: A review on the role of green processing technologies. Food and Bioprocess Technology, 15(9), 1948–1976. https://doi.org/10.1007/s11947-022-02812-5
Hassan, S. S., Williams, G. A., & Jaiswal, A. K. (2019). Moving towards the second generation of lignocellulosic biorefineries in the EU: Drivers, challenges, and opportunities. Renewable and Sustainable Energy Reviews, 101, 590–599. https://doi.org/10.1016/j.rser.2018.11.041
Huang, Y., Chiueh, P., Kuan, W., & Lo, S. (2016). Microwave pyrolysis of lignocellulosic biomass: Heating performance and reaction kinetics. Energy, 100, 137–144. https://doi.org/10.1016/j.energy.2016.01.088
Jeyavishnu, K., Thulasidharan, D., Shereen, M. F., & Arumugam, A. (2021). Increased revenue with high value-added products from cashew apple (anacardium occidentale L.)—Addressing global challenges. Food and Bioprocess Technology, 14(6), 985–1012. https://doi.org/10.1007/s11947-021-02623-0
Kiatphuengporn, S., Junkaew, A., Luadthong, C., Thongratkaew, S., Yimsukanan, C., Songtawee, S., & Faungnawakij, K. (2020). Roles of acidic sites in alumina catalysts for efficient d-xylose conversion to lactic acid. Green Chemistry, 22(24), 8572–8583. https://doi.org/10.1039/D0GC02573A
Kumar, R., & Wyman, C. E. (2013). Physical and chemical features of pretreated biomass that influence macro-/micro-accessibility and biological processing. In Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals (pp. 281–310). https://doi.org/10.1002/9780470975831.ch14
Lee, C. S., Conradie, A. V., & Lester, E. (2021). Review of supercritical water gasification with lignocellulosic real biomass as the feedstocks: Process parameters, biomass composition, catalyst development, reactor design and its challenges. Chemical Engineering Journal, 415, 128837. https://doi.org/10.1016/j.cej.2021.128837
Li, S., Deng, W., Li, Y., Zhang, Q., & Wang, Y. (2019). Catalytic conversion of cellulose-based biomass and glycerol to lactic acid. Journal of Energy Chemistry, 32, 138–151. https://doi.org/10.1016/j.jechem.2018.07.012
Licursi, D., Antonetti, C., Bernardini, J., Cinelli, P., Coltelli, M. B., Lazzeri, A., & Galletti, A. M. R. (2015). Characterization of the Arundo donax L. solid residue from hydrothermal conversion: Comparison with technical lignins and application perspectives. Industrial Crops and Products, 76, 1008–1024. https://doi.org/10.1016/j.indcrop.2015.08.007
Liu, G., Sun, J., Zhang, J., Tu, Y., & Bao, J. (2015). High titer l-lactic acid production from corn stover with minimum wastewater generation and techno-economic evaluation based on aspen plus modeling. Bioresource Technology, 198, 803–810. https://doi.org/10.1016/j.biortech.2015.09.098
Liu, W., Zhou, Z., Guo, Z., Wei, Z., Zhang, Y., Zhao, X., & Kong, L. (2022). Microwave-induced controlled-isomerization during glucose conversion into lactic acid over a sn-beta catalyst. Sustainable Energy & Fuels, 6(5), 1264–1268. https://doi.org/10.1039/D1SE01971F
Luo, H., Bao, L., Kong, L., & Sun, Y. (2017). Low temperature microwave-assisted pyrolysis of wood sawdust for phenolic rich compounds: Kinetics and dielectric properties analysis. Bioresource Technology, 238, 109–115. https://doi.org/10.1016/j.biortech.2017.04.030
Luterbacher, J. S., Martin Alonso, D., & Dumesic, J. A. (2014). Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chemistry, 16(12), 4816–4838. https://doi.org/10.1039/C4GC01160K
Ma, H., Tingelstad, P., & Chen, D. (2023). Lactic acid production by catalytic conversion of glucose: An experimental and techno-economic evaluation. Catalysis Today, 408, 2–8. https://doi.org/10.1016/j.cattod.2022.10.019
Mäki-Arvela, P., Simakova, I. L., Salmi, T., & Murzin, D. Y. (2014). Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chemical Reviews, 114(3), 1909–1971. https://doi.org/10.1021/cr400203v
Melgosa, R., Trigueros, E., Sanz, M. T., Cardeira, M., Rodrigues, L., Fernández, N., & Simões, P. (2020). Supercritical CO2 and subcritical water technologies for the production of bioactive extracts from sardine (Sardina pilchardus) waste. Journal of Supercritical Fluids. https://doi.org/10.1016/j.supflu.2020.104943
Melgosa, R., Sanz, M. T., & Beltrán, S. (2021). Supercritical CO2 processing of omega-3 polyunsaturated fatty acids – Towards a biorefinery for fish waste valorization. Journal of Supercritical Fluids. https://doi.org/10.1016/j.supflu.2020.105121
Moreno-González, M., & Ottens, M. (2021). A structured approach to recover valuable compounds from agri-food side streams. Food and Bioprocess Technology, 14(8), 1387–1406. https://doi.org/10.1007/s11947-021-02647-6
Mujtaba, M., Fernandes Fraceto, L., Fazeli, M., Mukherjee, S., Savassa, S. M., Araujo de Medeiros, G., & Vilaplana, F. (2023). Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. Journal of Cleaner Production, 402, 136815. https://doi.org/10.1016/j.jclepro.2023.136815
Nüchter, M., Ondruschka, B., Bonrath, W., & Gum, A. (2004). Microwave assisted synthesis – A critical technology overview. Green Chemistry, 6(3), 128–141. https://doi.org/10.1039/B310502D
Ögmundarson, Ó., Sukumara, S., Laurent, A., & Fantke, P. (2020). Environmental hotspots of lactic acid production systems. GCB Bioenergy, 12(1), 19–38. https://doi.org/10.1111/gcbb.12652
Pérez, E., Tuck, C. O., & Poliakoff, M. (2018). Valorisation of lignin by depolymerisation and fractionation using supercritical fluids and conventional solvents. The Journal of Supercritical Fluids, 133, 690–695. https://doi.org/10.1016/j.supflu.2017.07.033
Rivas-Vela, C., Amaya-Llano, S., Castaño-Tostado, E., & Castillo-Herrera, G. (2021). Protein hydrolysis by subcritical water: A new perspective on obtaining bioactive peptides. Molecules. https://doi.org/10.3390/molecules26216655
Ruiz, H. A., Galbe, M., Garrote, G., Ramirez-Gutierrez, D. M., Ximenes, E., Sun, S., & Ladisch, M. R. (2021). Severity factor kinetic model as a strategic parameter of hydrothermal processing (steam explosion and liquid hot water) for biomass fractionation under biorefinery concept. Bioresource Technology, 342, 125961. https://doi.org/10.1016/j.biortech.2021.125961
Saravanan, A., Yaashikaa, P. R., Kumar, P. S., Thamarai, P., Deivayanai, V. C., & Rangasamy, G. (2023). A comprehensive review on techno-economic analysis of biomass valorization and conversional technologies of lignocellulosic residues. Industrial Crops and Products, 200, 116822. https://doi.org/10.1016/j.indcrop.2023.116822
Silveira, M. S., Fontes, C. P. M. L., Guilherme, A. A., Fernandes, F. A. N., & Rodrigues, S. (2012). Cashew apple juice as substrate for lactic acid production. Food and Bioprocess Technology, 5(3), 947–953. https://doi.org/10.1007/s11947-010-0382-9
Sluiter, J. B., Ruiz, R. O., Scarlata, C. J., Sluiter, A. D., & Templeton, D. W. (2010). Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. Journal of Agricultural and Food Chemistry, 58(16), 9043–9053. https://doi.org/10.1021/jf1008023
Wang, Y., Deng, W., Wang, B., Zhang, Q., Wan, X., Tang, Z., & Wan, H. (2013). Chemical synthesis of lactic acid from cellulose catalysed by lead(II) ions in water. Nature Communications, 4(1), 2141. https://doi.org/10.1038/ncomms3141
Wang, Z., Zhu, M., Li, M., Wang, J., Wei, Q., & Sun, R. (2016). Comprehensive evaluation of the liquid fraction during the hydrothermal treatment of rapeseed straw. Biotechnology for Biofuels, 9(1), 142. https://doi.org/10.1186/s13068-016-0552-8
Xu, S., Wu, Y., Li, J., He, T., Xiao, Y., Zhou, C., & Hu, C. (2020). Directing the simultaneous conversion of hemicellulose and cellulose in raw biomass to lactic acid. ACS Sustainable Chemistry & Engineering, 8(10), 4244–4255. https://doi.org/10.1021/acssuschemeng.9b07552
Ye, J., Chen, C., Zheng, Y., Zhou, D., Liu, Y., Chen, D., & Wang, F. (2021). Efficient conversion of cellulose to lactic acid over yttrium modified siliceous beta zeolites. Applied Catalysis A: General. https://doi.org/10.1016/j.apcata.2021.118133
Zhang, W., Xu, S., Xiao, Y., Qin, D., Li, J., & Hu, C. (2021). The insights into the catalytic performance of rare earth metal ions on lactic acid formation from biomass via microwave heating. Chemical Engineering Journal, 421, 130014. https://doi.org/10.1016/j.cej.2021.130014
Zhu, Y., Romain, C., & Williams, C. K. (2016). Sustainable polymers from renewable resources. Nature, 540(7633), 354–362. https://doi.org/10.1038/nature21001
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the Agencia Estatal de Investigación (grant numbers TED2021-129311B-I00, PDC2022-133443-I00, and PID2022-136385OB-I00). A. Bermejo-López post-doctoral contract was funded by Margarita Salas grant (MARSA22/03). R. Melgosa contract was funded by a Beatriz Galindo Research Fellowship (BG20/00182).
Author information
Authors and Affiliations
Contributions
A. B. L: validation, methodology, investigation, and writing—original draft. A. E. I.: conceptualization, methodology, and visualization. R. M.: methodology. S. B.: methodology, data curation, supervision, funding acquisition, and writing—review and editing. M. T. S.: conceptualization, supervision, project administration, funding acquisition, and writing—review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bermejo-López, A., Illera, A.E., Melgosa, R. et al. Comparative Selective Conversion of Biomass-Derived Mono- and Polysaccharides into Lactic Acid with Lanthanide Lewis Acid Catalysts. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03416-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03416-x