Abstract
Purpose of Review
Diagnosis of autoimmune gastritis (AIG) is often delayed because of the absence of typical symptoms. Clinical guidelines are lacking which results in inadequate treatment and poor cancer screening. This review presents an overview of current management options and aims at raising awareness for this often-neglected disease.
Recent Findings
Autoimmune gastritis is mostly thought of as a disease of the elderly with vitamin B12 deficiency and pernicious anemia. Today it is recognized that AIG is found with a similar prevalence among all age-groups, with iron deficiency being a frequent feature. Conventional therapy consists of adequate iron and vitamin B12 supplementation as well as symptomatic approaches. The associated risk for gastric adenocarcinoma and gastric neuroendocrine tumors requires regular endoscopic follow up. Novel therapies aiming to reduce gastric atrophy and cancer risk are currently under development.
Summary
Treatment of autoimmune gastritis should focus on optimizing supplementation of deficiencies and include cancer prevention measures. Clinical research should address the possibility to arrest the inflammatory process and to prevent progression of AIG. International guidelines on management and endoscopic screening intervals should be set up.
Similar content being viewed by others
Introduction
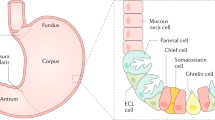
Autoimmune gastritis (AIG) is characterized by a chronic inflammation of the stomach fundus and corpus due to an autoimmune destruction of the acid-producing oxyntic mucosa [1, 2]. Specifically, anti-parietal cell antibodies (PCA) recognize a subunit of the proton-pump H+/K+-ATPase located on parietal cells, leading to a loss of these cells and finally resulting in mucosal atrophy. Since parietal cells are only present in the fundus and corpus, the antrum remains spared from inflammation and atrophy. Along with the destruction of parietal cells, acid secretion declines, resulting in hypochlorhydria, followed by achlorhydria [1, 2, 3]. Consequently, maldigestion, bacterial changes in the stomach, and small bowel and deficiencies in iron, as well as in vitamin B12 due to the concomitant loss of intrinsic factor, may occur [4, 5•]. Even though iron deficiency is the most prevalent, AIG is historically best known for pernicious anemia with underlying vitamin B12 deficiency, which may occur much later in the disease course [4].
The risk of developing gastric neoplasia is significantly elevated in AIG patients, since the persistent inflammation, with a predominance of lymphocytes, leads to gastric atrophy and may further lead to precancerous lesions [1, 6, 7, 8, 9]. Even though the exact cancer risk is still under some discussion, a meta-analysis of six European countries found an overall relative risk for gastric adenocarcinoma of 6.8% among AIG patients [9]. In addition, AIG is associated with an increased risk for type 1 gastric NETs, with an estimated incidence rate of 0.4–0.7% per-person year [6, 7, 10]. Physiologically, parietal cells are triggered to secrete acid upon stimulation with gastrin and histamine, produced by G-cells and enterochromaffin-like cells (ECL), respectively. The destruction of parietal cells may result in hyperplasia of G-cells and ECLs, with especially the latter at high risk for type 1 gastric NET transformation [6, 7, 10].
Treatment of AIG relies on supplementation of iron and vitamin B12, as well as symptomatic approaches to ameliorate gastrointestinal (GI) complaints [1]. Immunosuppressive therapy is currently not part of the therapeutic management; however new formulations are under development and may find their application into clinical practice. Endoscopic follow-up is critical for early detection of premalignant and early neoplastic lesions in the stomach, which needs to be addressed in international guidelines.
Furthermore, AIG is associated with other autoimmune disorders, including Hashimoto’s autoimmune thyroiditis with an especially high prevalence of 10–40%, but also diabetes mellitus type I, vitiligo, Addison’s disease, and non-alcoholic steatohepatitis, which should be accounted for when AIG patients present with suggestive symptoms [1, 11, 12, 13].
Epidemiology and Background
The prevalence of AIG is estimated to range from 0.5 to 4.5% globally, affecting predominantly women [1, 14]. However, epidemiologic data is scarce, and it seems likely that prevalence has been underestimated in the past, with some more recent studies estimating the prevalence to be up to 7.8–19.5% [3, 15]. Since GI symptoms are usually unspecific and mild, AIG might often not be suspected. Especially in young women with iron deficiency, AIG may often be overlooked—in fact, the current AGA guideline on Gastrointestinal Evaluation of Iron Deficiency Anemia advises against screening for AIG in premenopausal women, contributing to a low detection rate [16•]. In order to provide treatment recommendations in this review, an update in manifestations and diagnostics is first introduced to help increase AIG detection rate.
Iron deficiency is highly prevalent in AIG, with 25–50% of patients suffering from microcytic hypochromic anemia, due to an ineffective iron resorption in AIG [1, 3, 4]. Physiologically, 50% of our daily iron intake, is absorbed as non-heme iron and must be reduced to its ferrous form (Fe2+) to be biologically available [17]. This chemical state is primarily obtained in acidic pH states, explaining why iron absorption is impaired in AIG [18, 19].
Pernicious anemia typically occurs later in the disease course and manifests as hyperchromic macrocytic anemia and neurological symptoms in late stages [4]. In the past, AIG and pernicious anemia have often been used as interchangeable terms, although pernicious anemia only occurs in about 15–20% of AIG patients [1, 3]. Vitamin B12 deficiency is not only due to a loss of intrinsic factor, which is produced by parietal cells, but also due to the loss of gastric acid, which releases vitamin B12 from food sources. The typically late occurrence of vitamin B12 deficiency can be explained by a low daily turnover (2–3 ug per day) versus a large store of vitamin B12 (2–5 mg), which can last for years [1, 20].
Manifestation and Symptoms
The clinical presentation of AIG is usually unspecific and most often presents with mild or subtle symptoms [1, 2]. Patients may present with symptoms of anemia, but also with GI symptoms like epigastric pain, postprandial fullness, or nausea (Table 1). Several mechanisms are involved and contribute to GI symptoms in AIG. Often lower GI tract symptoms, similar to irritable bowel syndrome (IBS) with bloating and altered colonic transit have been reported, likely explained by an altered microbiome composition in AIG [5•, 21].
A recent interesting analysis of GI symptoms in Italy has shown that about 56% of AIG patients complain of GI symptoms, of which 70% have upper GI symptoms only. Postprandial distress-like dyspepsia was the most common complaint, with about 60% of symptomatic patients suffering of it, followed by reflux-like symptoms and epigastric pain. Lower GI tract symptoms were also reported, such as abdominal pain, constipation, and diarrhea [5•]. However, a systematic approach to determine symptom severity in AIG is currently lacking. To overcome this drawback, a new AIG symptom score is under development by this study group and currently evaluated among AIG patients to better determine common symptoms and objectify symptom severity in AIG.
Predominant and characteristic symptoms of AIG are deficiencies in iron and less commonly vitamin B12. Both these deficiencies result in anemia (micro- or macrocytic, depending on origin), accompanied by fatigue, shortness of breath, hair loss, weakness, or sleeping disorder.
Furthermore, prolonged vitamin B12 deficiency leads to degradation of central and peripheral nerves, since the synthesis of succinyl coenzyme A, which is required for the myelinization of nervous shafts, depends on the availability of vitamin B12. The sometimes irreversible symptoms of vitamin B12 deficiency may be as severe as peripheral polyneuropathy, paresthesia, cognitive impairment, and psychological disorders up to psychosis [20].
Management and therapy
Diagnosis
Patients with iron deficiency, anemia, and/or unspecific GI symptoms should be evaluated for AIG. The laboratory work-up should include a blood count, iron status (ferritin, transferrin, transferrin saturation), vitamin B12 status, and the specific AIG serologic markers PCA, intrinsic factor-antibodies (IFA), and if available gastrin. According to a study including 165 patients with histologically confirmed AIG, 81% of patients were positive for PCA, whereas approximately 10% of dyspeptic or iron deficient controls were false positive [22]. In a retrospective analysis of patients with iron deficiency of unknown etiology, PCA levels with a cut-off of > 100 U/ml were found to be highly specific (98%) and sensitive (93%) to diagnose AIG correctly [23]. IFA are far less common in AIG patients, with a prevalence of 27%, and may be positive in combination with PCA or by itself [22]. Gastrin levels are elevated dependent on the level of atrophy, since gastrin is the key trigger for acid production [1]. Additionally, the determination of pepsinogen I (secreted by oxyntic mucosa) and II (secreted by antral mucosa) may be helpful for non-invasive graduation of atrophy, since pepsinogen I is distinctively decreased in oxyntic atrophy while pepsinogen II remains unaffected [1, 3]. A low pepsinogen I:II ratio therefore is an indicator for atrophy. Gastrin, pepsinogen I and pepsinogen II, together with H. pylori antibodies, have been incorporated in panel of markers (GastroPanel™), which is being used for non-invasive diagnosis of atrophic gastritis and H. pylori infection in dyspeptic patients before endoscopy [24]. Indeed, in patients with dyspepsia but without any alterations in these serologic markers, AIG seems to be unlikely [25].
Endoscopically, AIG is very difficult to distinguish from healthy mucosa, especially in early stages. Acute inflammation may be present in the florid stage of AIG, with endoscopically flattened gastric corpus folds or swelling and reddening of the mucosa [1, 2, 26••]. In the subsequent stage, atrophy of the corpus and fundus may be visible with pale mucosa and translucent vessels due to thinned mucosa, while the antrum remains unaffected (Fig. 1) [26••]. Intestinal metaplasia may be recognized endoscopically with white opaque fields and blueish crests [26••]. Endoscopic biopsies should be taken accordingly to the updated Sydney system, which includes two samples from the antrum, besides two biopsies from the body and one from the incisura angularis [27••] with separate sampling and biopsies of any suspicious regions.
Measuring gastric pH during endoscopy could be a helpful and affordable tool to help identify AIG; however, currently, it is not an established method. High pH levels can indicate loss of function of parietal cells, which may already be present in early stages of AIG, when histopathological changes are not distinct yet [28]. In a study using pH test stripes during endoscopy, all evaluated AIG patients were found to have achlorhydria (pH levels > 6) or at least hypochlorhydria (pH 3–5) [23]. An integratable device for gastroscopes, EndoFaster™, has recently been developed to measure gastric pH in real-time during endoscopy, in order to identify areas of atrophy more efficiently [29]. Nonetheless, criticism has been raised since there is currently no validated method available to measure gastric pH during endoscopy and because the measured results might not reflect physiological levels.
As intake of proton pump inhibitors also raises gastric pH, this must be excluded when pH is tested [28].
Histology remains to be the gold standard for diagnosis of AIG. Histopathological signs are restricted to the oxyntic mucosa and therefore the gastric corpus and fundus. Possible findings include immune infiltrates, atrophy, and intestinal or pseudopyloric metaplasia. In the early stage of AIG, dense immune cell infiltrates may be present along with a patchy destruction of parietal cells, which may be difficult to distinguish from other types of gastritis. As disease progresses, atrophy of oxyntic mucosa and pseudopyloric metaplasia becomes apparent. In the end stage, oxyntic mucosa is often completely destroyed, leaving intestinal metaplasia and possibly enterochromaffin-like hyperplasia [2]. Histologically, atrophy should be graded using the scoring system Operative Link on Gastritis Assessment (OLGA), ranging from stadium 0 (no atrophy) to IV (severe atrophy) [30]. OLGA allows stratification of patients depending on their risk for gastric neoplasia and can therefor help detect patients at risk [30, 31]. In some cases, H. pylori may be present concomitantly with AIG, making it impossible to diagnose AIG with certainty. Until today is incompletely understood, if H. pylori might be able to induce AIG in susceptible individuals and how co-occurrence affects disease course [1]. Even so, eradication of H. pylori is indicated and in individuals with suspected underlying AIG, re-endoscopy 3 to 6 months after eradication should be performed [26••].
As AIG is often associated with Hashimoto’s thyroiditis, with a prevalence of up to 40% among AIG patients [11, 12], routine TSH measurements might be useful in patients diagnosed with AIG.
Treatment
Currently, no causative treatment for AIG exists. However, symptomatic measures should be taken to alleviate concomitant symptoms. These include the screening and supplementation of deficiencies in iron and vitamin B12 as well as treatment of unspecific GI symptoms.
Furthermore, chronic atrophic gastritis as a consequence of AIG implicates an elevated risk for gastric malignancy, specifically for neuroendocrine tumors (NETs) and gastric adenocarcinoma [1, 9, 32••]. Therefore, regular surveillance endoscopies are critical; however, guidelines on the recommended intervals are inconsistent.
The therapeutic management of AIG is described in detail in Table 2.
General Medication
In patients with dyspepsia or feeling of fullness, common medications for functional dyspepsia may be used (e.g., prokinetics). PPIs have no role in AIG treatment due to preexisting hypochlorhydria and do not alleviate reflux-like symptoms, which are typically functional and not associated with endoscopic findings of gastroesophageal reflux disease [33•, 34]. Even though this seems logical when understanding the pathophysiologic mechanism, PPIs are often prescribed to AIG patients. It is worth mentioning, however, that in atrophic gastritis some 20–24% of patients may complain of GERD-like symptoms [35, 36, 37], suggesting that acid is not the only symptom trigger.
AIG patients may suffer from bloating and altered colonic transit time partially due to small intestine bacterial overgrowth (SIBO). If SIBO is suspected in patients, testing with lactulose hydrogen breath test should be performed for verification [38]. Treatment with rifaximin may be considered to reduce intestinal bacterial load, which has been shown effective in a SIBO cohort according to meta-analysis [39]. The use of probiotics has been reported for the prevention and treatment of SIBO and may be considered in symptomatic individuals [40].
Diet
GI symptoms like dyspepsia, postprandial fullness, and bloating might be influenced by diet. As in other gastric disorders, smaller meals which are evenly distributed throughout the day should be recommended. Especially high protein-containing meals may be difficult to digest and more prone to causing GI symptoms. Generally, patients should be advised on a healthy diet rich in vegetables, grains, and fruits. However, different ingredients may aggravate GI symptoms on an individual level in some patients, which should be taken into account.
Deficiencies and Supplementation
Iron Deficiency
Due to the high prevalence of iron deficiency (25–50% of AIG patients), correcting iron supplementation is a crucial part of AIG management. As oral iron is poorly absorbed in AIG, supplementation should be parenteral. Available preparations include iron sucrose, iron gluconate, iron carboxymaltose, iron isomaltoside, and low molecular weight iron dextran, which are either administered in a single or multiple doses according to product recommendation and severity of deficiency [41]. Hemoglobin increase should be monitored during treatment; if there is no increase, other causes of anemia should be re-evaluated. Iron status should be performed 4–6 weeks after the last infusion as ferritin levels may be falsely elevated shortly after treatment. As AIG is a chronic disease, iron parameters should be monitored in regular intervals for early detection of recurrent deficiency.
Vitamin B12 Deficiency
Oral vitamin B12 preparations are ineffective in AIG since intrinsic factor is absent. If vitamin B12 deficiency is present, parenteral substitution should be performed. 1000 μg cyanocobalamin three times a week intramuscularly is recommended for 2 weeks or until normalization of vitamin B12 levels. As with iron parameters, patients should be regularly screened for vitamin B12 deficiency, and substitution should be performed as needed.
Other Deficiencies
Few small studies have reported a higher prevalence of deficiencies in vitamin C, vitamin D, and calcium [42], but data are very limited.
Both, vitamin D deficiency and hypocalcemia may lead to osteoporosis. No studies that have examined the prevalence of osteoporosis in AIG cohorts exist. However, chronic atrophic gastritis was shown to be correlated with osteoporosis in a study of 401 postmenopausal women [43].
Regular screening and consequent substitution of vitamin D and/or calcium may be useful. So far, there is no evidence of an advantage of regular vitamin C substitution.
Cancer Surveillance
Several studies have shown an increased risk for gastric adenocarcinoma and type 1 gastric neuroendocrine tumors (NETs) [6, 9, 44•]. Especially patients with severe atrophy (OLGA stadiums III–IV) or with concomitant H. pylori infection seem to be at higher risk for gastric malignancies [45]. However, there has been some controversy on this topic, since many studies were either short-lived or had small patient numbers, leading to inconclusive results. Because of this, European and American guidelines have different positions on screening for gastric neoplasia in AIG, with Europeans recommending regular screening intervals, an advice not yet included in the American guidelines [27••, 46••].
In 2019, a shared European guideline on the Management of epithelial precancerous conditions and lesions in the stomach (MAPS II) was published [27••]. For the first time, specific screening recommendations for patients with AIG were made. A screening interval of 3 to 5 years was recommended in all patients with AIG [27••]. Furthermore, MAPS II recommended—for patients with severe atrophic gastritis or a positive family history of gastric cancer—to be endoscopically followed-up every 1 to 2 years. High-definition endoscopy with chromoendoscopy should be used in order to detect pre- and neoplastic lesions. Biopsies of at least two sites from the antrum and the corpus and one from the incisura angularis, as well as separate biopsies from suspicious lesions, should be taken [27••]. No specific recommendation for the follow-up interval in patients with intestinal metaplasia was given.
The American Gastroenterological Association (AGA) provides three relevant guidelines, with each addressing AIG more or less directly. In 2020, the guideline on Gastrointestinal Evaluation of Iron Deficiency Anemia distanced itself from gaining biopsies to diagnose atrophic gastritis either due to AIG or H. pylori, arguing that diagnosis would not affect patients’ outcomes and not result in causative treatment [16•]. However, due to the elevated risk for gastric neoplasia in AIG, these recommendations could lead to a late diagnosis of atrophy or metaplasia, with the risk of missing preneoplastic stages proceeding malignancy [6, 7, 9, 10]. Moreover, the Clinical Practice Guideline on Management of Gastric Intestinal Metaplasia published in 2020 advised against endoscopic surveillance in patients with intestinal metaplasia, even though acknowledging that AIG was beyond the scope of the guideline [46••].
Currently, the only AGA statement specifically on surveillance in AIG is not included in an official guideline, but expressed in the AGA expert review: Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis [26••]. Even though this update discusses atrophic gastritis in general, it does address AIG-induced atrophy specifically. The authors generally propose an endoscopic interval of three years for patients with advanced atrophic gastritis [26••]. It is also acknowledged that an optimal surveillance interval for AIG is unknown and therefore individual assessment is necessary. The screening for type 1 gastric NETs is explicitly recommended in AIG patients, and a follow-up endoscopy every 1 to 2 years should be performed if NET has been diagnosed and removed in the past [26••].
AIG Therapy: a Look to the Future
At the moment no causal treatment for AIG is available. New treatment options to reduce gastric inflammation and thereby prevent the development and progression of atrophy, metaplasia and lastly gastric neoplasia are eagerly anticipated.
A recent open study found that L-cysteine (300 mg daily for 1 year) was able to provide a recovery in gastric function, assessed by using validated biomarkers [47]. These promising results need to be confirmed in a large, double-blind trial.
Immunosuppressive agents have only scarcely been evaluated in AIG. In the 1960s, two small clinical trials have demonstrated that regeneration of oxyntic mucosa and restored vitamin B12 uptake can be achieved with systemic corticosteroids [48, 49]. However, due to the many undesirable effects of long-term systemic corticosteroid use, steroids have not found their way into clinical use. However, a locally acting anti-inflammatory agent, restricting its effect to the gastric mucosa, is under development by Marinomed Biotech AG (Korneuburg, Austria). The company has announced to be working on a new technology which allows dissolving otherwise difficult substances for local bioavailability and thereby avoiding systemic effects. However, development is still pre-clinical.
Since type 1 NETs are gastrin-dependent tumors, CCK2-receptor antagonists (namely netazepide) normalize tumor biomarkers and cause tumor regression [50]. A more recent study found that this drug, given continuously to patients with AIG, can eradicate gastric neuroendocrine tumor, an effect followed by tumor regrow after stopping treatment [51]. These results suggest that CCK2-receptor antagonists may be a potential medical and targeted treatment for type 1 gastric NETs and an alternative to regular endoscopy or surgery.
Conclusions
To conclude, AIG usually presents with unspecific symptoms and deficiencies in iron and/or vitamin B12, leading to a delayed diagnosis and reduction in quality of life. Treatment of AIG currently encompasses substitution of underlying deficiencies and most importantly screening for gastric neoplasia, since AIG is associated with an increased risk for type 1 gastric NET and gastric adenocarcinoma. The most recent clinical guidelines provide limited recommendations on endoscopic screening, with intervals ranging between 1 and 5 years depending on guideline and previous histologic findings. In order to provide better care for AIG patients, larger clinical trials are required to better define symptoms, examine cancer risk, and establish causative treatment options.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lenti MV, Rugge M, Lahner E, Miceli E, Toh B-H, Genta RM, De Block C, Hershko C, Di Sabatino A. Autoimmune gastritis. Nat Rev Dis Primers. 2020. https://doi.org/10.1038/s41572-020-0187-8.
Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis—pathogenesis, pathology and Management. Nat Rev Gastroenterol Hepatol. 2013;10:529–41.
Kulnigg-Dabsch S. Autoimmune gastritis. Wien Med Wochenschr. 2016;166:424–30.
Hershko C, Ronson A, Souroujon M, Maschler I, Heyd J, Patz J. Variable hematologic presentation of autoimmune gastritis: age-related progression from iron deficiency to cobalamin depletion. Blood. 2006;107(4):1673–9.
• Carabotti M, Lahner E, Esposito G, Sacchi MC, Severi C, Annibale B. Upper gastrointestinal symptoms in autoimmune gastritis. Medicine. 2017. https://doi.org/10.1097/md.0000000000005784. Important objective evaluation of GI symptoms in AIG.
Annibale B, Azzoni C, Corleto VD, Di Giulio E, Caruana P, D’Ambra G, et al. Atrophic body gastritis patients with enterochromaffin-like cell dysplasia are at increased risk for the development of type I gastric carcinoid. Eur J Gastroenterol Hepatol. 2001;13(12):1449–56.
Vannella L, Sbrozzi-Vanni A, Lahner E, Bordi C, Pilozzi E, Corleto VD, et al. Development of type i gastric carcinoid in patients with chronic atrophic gastritis. Aliment Pharmacol Ther. 2011;33(12):1361–9.
Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988Jul 1;48(13):3554–60.
Vannella L, Lahner E, Osborn J, Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther. 2013;37(4):375–82.
Lahner E, Esposito G, Pilozzi E, Purchiaroni F, Corleto VD, Di Giulio E, et al. Occurrence of gastric cancer and carcinoids in atrophic gastritis during prospective long-term follow up. Scand J Gastroenterol. 2015;50(7):856–65.
Cellini M, Santaguida MG, Virili C, Capriello S, Brusca N, Gargano L, Centanni M. Hashimoto’s thyroiditis and autoimmune gastritis. Front Endocrinol. 2017. https://doi.org/10.3389/fendo.2017.00092.
Centanni M, Marignani M, Gargano L, Corleto VD, Casini A, Delle Fave G, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159(15):1726–30.
Kawanaka M, Tanikawa T, Kamada T, Ishii K, Urata N, Nakamura J, et al. High prevalence of autoimmune gastritis in patients with nonalcoholic steatohepatitis. Intern Med. 2019;58(20):2907–13.
Zhang Y, Weck MN, Scḧottker B, Rothenbacher D, Brenner H. Gastric parietal cell antibodies, helicobacter pylori infection, and chronic atrophic gastritis: evidence from a large population-based study in germany. Cancer Epidemiol Biomarkers Prev. 2013;22(5):821–6.
Wang S-M, Roth MJ, Murphy GA, Dawsey SM, Fan J-H, Taylor PR, Qiao Y-L, Abnet CC. Serologic profile of antiparietal cell antibodies, pepsinogens, and H. pylori and risk of upper gastrointestinal cancer: a nested case–control study in China. Cancer Epidemiol Biomark Prev. 2019;28:2022–9.
• Ko CW, Siddique SM, Patel A, Harris A, Sultan S, Altayar O, et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology. 2020;159(3):1085–94. https://doi.org/10.1053/j.gastro.2020.06.046. Guideline on anemia and work-up of patients. AIG patients are only imcompletely represented in the guideline recommendations.
Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, et al. Identification of an intestinal heme transporter. Cell. 2005;122(5):789–801.
Betesh AL, Santa Ana CA, Cole JA, Fordtran JS. Is achlorhydria a cause of iron deficiency anemia?1. Am J Clin Nutr. 2015;102(1):9–19.
Skikne BS, Lynch SR, Cook JD. Role of gastric acid in food iron absorption. Gastroenterology. 1981;81(6):1068–71. https://doi.org/10.1016/S0016-5085(81)80013-3.
Mohamed M, Thio J, Thomas RS, Phillips J. Pernicious anaemia. BMJ; 2020. p. m1319.
Parsons BN, Ijaz UZ, D’Amore R, et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLOS Pathogens. 2017;13(11):e1006653. https://doi.org/10.1371/journal.ppat.1006653.
Lahner E, Norman GL, Severi C, Encabo S, Shums Z, Vannella L, et al. Reassessment of intrinsic factor and parietal cell autoantibodies in atrophic gastritis with respect to cobalamin deficiency. Am J Gastroenterol. 2009;104(8):2071–9.
Kulnigg-Dabsch S, Resch M, Oberhuber G, Klinglmueller F, Gasche A, Gasche C. Iron deficiency workup reveals high incidence of autoimmune gastritis with parietal cell antibody as reliable screening test. Semin Hematol. 2018;55(4):256–61. https://doi.org/10.1053/j.seminhematol.2018.07.003.
Koivurova OP, Koskela R, Blomster T, et al. Serological biomarker panel in diagnosis of atrophic gastritis and Helicobacter pylori infection in gastroscopy referral patients: clinical validation of the new-generation gastropanel®test. Anticancer Res. 2021;41:5527–37.
Yang YX, Brill J, Krishnan P, Leontiadis G. American Gastroenterological Association Institute Guideline on the role of upper gastrointestinal biopsy to evaluate dyspepsia in the adult patient in the absence of visible mucosal lesions. Gastroenterology. 2015;149(4):1082–7.
•• Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology [Internet]. 2021;161(4):1325-1332.e7. https://doi.org/10.1053/j.gastro.2021.06.078. No American guideline on management of AIG patients currently exists, however this expert review comes close to it and offers advice, especially on screening for neoplasia.
•• Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Port. Endoscopy. 2019;51(4):365–88. The only guideline on screening managment of AIG patients currently available.
Ghosh T, Lewis DI, Axon ATR, Everett SM. Review article: methods of measuring gastric acid secretion. Aliment Pharmacol Ther. 2011;33(7):768–81.
Zullo A, Germanà B, Galliani E, Khalaf K, Hassan C, Monica F. Real-time determination of gastric juice pH with EndoFaster® for atrophic gastritis assessment. Dig Liver Dis. 2022;54(12):1646–8.
Rugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L, De Pretis G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56(5):631–6.
Rugge M, Correa P, Di Mario F, El-Omar E, Fiocca R, Geboes K, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40(8):650–8.
•• Weise F, Vieth M, Reinhold D, Haybaeck J, Goni E, Lippert H, et al. Gastric cancer in autoimmune gastritis: a case-control study from the German centers of the staR project on gastric cancer research. United Eur Gastroenterol J. 2020;8(2):175–84. A current study on the risk of gastric neopalsia in AIG.
•• Gomez-Cifuentes JD, Jordan-Sparkman DYG. Management of upper gastrointestinal symptoms in patients with autoimmune gastritis. Curr Opin Gastroenterol. 2022;38(6):600–6. Often management of GI symptoms in AIG is neglected, here advice on this matter is provided.
Pilotto V, Maddalo G, Orlando C, Fassan M, Rugge M, Farinati F, et al. Objective evidence of gastro-esophageal reflux disease is rare in patients with autoimmune gastritis. J Gastrointest Liver Dis. 2021;30(1):30–6.
Miceli E, Lenti MV, Padula D, Luinetti O, Vattiato C, Monti CM, et al. Common features of patients with autoimmune atrophic gastritis. Clin Gastroenterol Hepatol. 2012;10(7):812–4.
Carabotti M, Esposito G, Lahner E, Pilozzi E, Conti L, Ranazzi G, Severi C, Bellini M, Annibale B. Gastroesophageal reflux symptoms and microscopic esophagitis in a cohort of consecutive patients affected by atrophic body gastritis: a pilot study. Scand J Gastroenterol. 2019;54:35–40.
Tenca A, Massironi S, Pugliese D, Consonni D, Mauro A, Cavalcoli F, et al. Gastro-esophageal reflux and antisecretory drugs use among patients with chronic autoimmune atrophic gastritis: a study with pH-impedance monitoring. Neurogastroenterol Motil. 2016;28(2):274–80.
Kužela L. Small intestinal bacterial overgrowth syndrome. Gastroenterol a Hepatol. 2015;69(1):70–2.
Gatta L, Scarpignato C, McCallum RW, Lombardo L, Pimentel M, D’Incà R, et al. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther. 2017;45(5):604–16.
Zhong C, Qu C, Wang B, Liang S, Zeng B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth. J Clin Gastroenterol. 2017;51(4):300–11.
Auerbach M, Deloughery T. Single-dose intravenous iron for iron deficiency: a new paradigm. Hematology. 2016;2016(1):57–66.
Cavalcoli F, Zilli A, Conte D, Massironi S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: a review. World J Gastroenterol. 2017;23(4):563–72.
Kim HW, Kim YH, Han K, Nam GE, Kim GS, Han BD, et al. Atrophic gastritis: a related factor for osteoporosis in elderly women. PLoS ONE. 2014;9(7):5–9.
•• Rugge M, Genta RM, Malfertheiner P, Graham DY. Steps forward in understanding gastric cancer risk. Gut. 2022;2022:328514. https://doi.org/10.1136/gutjnl-2022-328514. An important review on AIG cancer risk.
Rugge M, Fassan M, Pizzi M, Zorzetto V, Maddalo G, Realdon S, et al. Autoimmune gastritis: histology phenotype and OLGA staging. Aliment Pharmacol Ther. 2012;35(12):1460–6.
•• Gupta S, Li D, El Serag HB, Davitkov P, Altayar O, Sultan S, et al. AGA clinical practice guidelines on management of gastric intestinal metaplasia. Gastroenterology. 2020;158(3):693–702. https://doi.org/10.1053/j.gastro.2019.12.003. Guidelines on the management of intestinal metaplasia, which is a common precrusor lesion in AIG.
Di Mario F, Rodriguez-Castro KI, Franceschi M, Landi S, Grillo S, Franzoni L, Russo M, Brandimarte G, Tursi A, Crafa P. Improvement of symptoms in patients affected by chronic atrophic gastritis using L-cysteine [acetium®]. Digestive Diseases. (2022) https://doi.org/10.1159/000528168
Jeffries GH, Todd JE, Sleisenger MH. The effect of prednisolone on gastric mucosal histology, gastric secretion, and vitamin B 12 absorption in patients with pernicious anemia. J Clin Invest. 1966;45(5):803–12.
Wall AJ, Whittingham S, Mackay IR, Ungar B. Prednisolone and gastric atrophy. Clin Exp Immunol. 1968;3(4):359–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4871897%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1578917 .
Moore AR, Boyce M, Steele IA, Campbell F, Varro A, Pritchard DM. Netazepide, a gastrin receptor antagonist, normalises tumour biomarkers and causes regression of type 1 gastric neuroendocrine tumours in a nonrandomised trial of patients with chronic atrophic gastritis. PLoS ONE. 2013;8(10):1–12.
Boyce M, Moore AR, Sagatun L, Parsons BN, Varro A, Campbell F, Fossmark R, Waldum HL, Pritchard DM. Netazepide, a gastrin/cholecystokinin-2 receptor antagonist, can eradicate gastric neuroendocrine tumours in patients with autoimmune chronic atrophic gastritis. Br J Clin Pharmacol. 2016;83:466–75.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Orgler has a patent for a topical immunosuppressive agent for AIG licensed, which was sold to Marinomed Biotech AG. Dr. Orgler reports no other relevant financial activities outside the submitted work.
Dr. Dabsch has a patent for a topical immunosuppressive agent for AIG licensed, which was sold to Marinomed Biotech AG. Dr. Dabsch reports no other relevant financial activities outside the submitted work.
Dr. Malfertheiner reports personal fees from Aboca, personal fees from Bayer, personal fees from Biocodex, personal fees from Biohit, personal fees from Cinclus, personal fees from Imevax, personal fees from Malesci, personal fees from Menarini, and personal fees from Phatom, outside the submitted work.
Dr. Schulz has nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stomach
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Orgler, E., Dabsch, S., Malfertheiner, P. et al. Autoimmune Gastritis: Update and New Perspectives in Therapeutic Management. Curr Treat Options Gastro 21, 64–77 (2023). https://doi.org/10.1007/s11938-023-00406-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-023-00406-4