Opinion statement
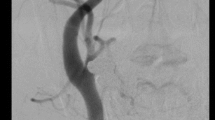
Takayasu arteritis, fibromuscular dysplasia (FMD), spontaneous arterial dissection, Raynaud’s phenomenon, and chilblains are vascular conditions that are associated with an increased predisposition in women and are often underdiagnosed. Takayasu arteritis has an incidence rate of 2.6 cases per million individuals per year in the USA and predominantly affects women of childbearing age. HLA-B5 genetic locus is linked with Takayasu arteritis susceptibility. Methods to determine active disease are limiting; currently utilized clinical and imaging findings and laboratory tests are of limited value for this purpose. Pregnancy poses risks for maternal and fetal complications, and these patients need additional monitoring and care before and after conception. Controlling hypertension and immunosuppression using steroids, biological and non-biological immunosuppressants, are key components of managing patients with this arteritis. FMD commonly affects middle-aged, white females. Its true prevalence is unknown. Renal and cerebrovascular beds are the most frequently involved vascular beds. Its clinical presentation varies from no symptoms to catastrophic events. Controlling vascular risk factors, periodic surveillance, and revascularization when indicated are important factors in FMD management. Spontaneous arterial dissections are less common, but are an important cause of morbidity and mortality in specific populations. Cervicocephalic dissection causes 10–20% of the strokes in young adults, and coronary artery dissection is the culprit in almost one fourth of young women presenting with acute myocardial infarction. Early diagnosis is key to improving prognosis in these patients, as the majority of patients have spontaneous resolution of the dissection with conservative management alone. Increased clinician awareness of the presentation features and angiographic findings are imperative for early diagnosis. Raynaud’s phenomenon and chilblains are cold- or stress-induced cutaneous lesions, commonly involving distal extremities. Secondary causes such as connective tissue diseases and malignancies must be thoroughly excluded during evaluation of these conditions. Cold avoidance, systemic and local warming, and oral vasodilator therapy are the mainstays of therapy.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
George J, Rapsomaniki E, Pujades-Rodriguez M, Shah AD, Denaxas S, Herrett E, et al. How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1,937,360 people. Circulation. 2015;132(14):1320–8.
Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123(11):1243–62.
Mosca L, Manson JE, Sutherland SE, Langer RD, Manolio T, Barrett-Connor E. Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1997;96(7):2468–82.
Hussain MA, Lindsay TF, Mamdani M, Wang X, Verma S, Al-Omran M. Sex differences in the outcomes of peripheral arterial disease: a population-based cohort study. CMAJ Open. 2016;4(1):E124–31.
Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919–29.
Isohisa I, Numano F, Maezawa H, Sasazuki T. HLA-Bw52 in Takayasu disease. Tissue Antigens. 1978;12(4):246–8.
Terao C. Revisited HLA and non-HLA genetics of Takayasu arteritis—where are we? J Hum Genet. 2016;61(1):27–32.
Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–34.
Eichhorn J, Sima D, Thiele B, Lindschau C, Turowski A, Schmidt H, et al. Anti-endothelial cell antibodies in Takayasu arteritis. Circulation. 1996;94(10):2396–401.
Ishihara T, Haraguchi G, Kamiishi T, Tezuka D, Inagaki H, Isobe M. Sensitive assessment of activity of Takayasu’s arteritis by pentraxin3, a new biomarker. J Am Coll Cardiol. 2011;57(16):1712–3.
Tamura N, Maejima Y, Tezuka D, Takamura C, Yoshikawa S, Ashikaga T, et al. Profiles of serum cytokine levels in Takayasu arteritis patients: Potential utility as biomarkers for monitoring disease activity. J Cardiol. 2017;7(3):278–85.
Chrapko BE, Chrapko M, Nocun A, Stefaniak B, Zubilewicz T, Drop A. Role of 18F-FDG PET/CT in the diagnosis of inflammatory and infectious vascular disease. Nucl Med Rev Cent East Eur. 2016;19(1):28–36.
Alibaz-Oner F, Dede F, Ones T, Turoglu HT, Direskeneli H. Patients with Takayasu’s arteritis having persistent acute-phase response usually have an increased major vessel uptake by 18F-FDG-PET/CT. Mod Rheumatol. 2015;25(5):752–5.
Magnoni M, Dagna L, Coli S, Cianflone D, Sabbadini MG, Maseri A. Assessment of Takayasu arteritis activity by carotid contrast-enhanced ultrasound. Circ Cardiovasc Imaging. 2011;4(2):e1–2.
Giordana P, Baque-Juston MC, Jeandel PY, Mondot L, Hirlemann J, Padovani B, et al. Contrast-enhanced ultrasound of carotid artery wall in Takayasu disease: first evidence of application in diagnosis and monitoring of response to treatment. Circulation. 2011;124(2):245–7.
Germano G, Macchioni P, Possemato N, Boiardi L, Nicolini A, Casali M, et al. Contrast-enhanced ultrasound of the carotid artery in patients with large vessel vasculitis: correlation with positron emission tomography findings. Arthritis Care Res (Hoboken). 2017;69(1):143–9.
Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. 1996;54(Suppl):S155–63.
• Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of Takayasu arteritis. Arthritis Rheumatol. 2017;69(4):846–53. This is the first randomized controlled trial of a treatment for Takayasu arteritis. All other treatment studies prior to this were observational in nature.
Kotter I, Henes JC, Wagner AD, Loock J, Gross WL. Does glucocorticosteroid-resistant large-vessel vasculitis (giant cell arteritis and Takayasu arteritis) exist and how can remission be achieved? A critical review of the literature. Clin Exp Rheumatol. 2012;30(1 Suppl 70):S114–29.
Labarca C, Makol A, Crowson CS, Kermani TA, Matteson EL, Warrington KJ. Retrospective comparison of open versus endovascular procedures for Takayasu arteritis. J Rheumatol. 2016;43(2):427–32.
Suri V, Aggarwal N, Keepanasseril A, Chopra S, Vijayvergiya R, Jain S. Pregnancy and Takayasu arteritis: a single centre experience from North India. J Obstet Gynaecol Res. 2010;36(3):519–24.
Comarmond C, Mirault T, Biard L, Nizard J, Lambert M, Wechsler B, et al. Takayasu arteritis and pregnancy. Arthritis Rheumatol. 2015;67(12):3262–9.
Assad AP, da Silva TF, Bonfa E, Pereira RM. Maternal and neonatal outcomes in 89 patients with Takayasu arteritis (TA): comparison before and after the TA diagnosis. J Rheumatol. 2015;42(10):1861–4.
Singh N, Tyagi S, Tripathi R, Mala YM. Maternal and fetal outcomes in pregnant women with Takayasu aortoarteritis: does optimally timed intervention in women with renal artery involvement improve pregnancy outcome? Taiwan J Obstet Gynecol. 2015;54(5):597–602.
• Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, Gray BH, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation. 2012;125(25):3182–90. This is the first publication from the US FMD Registry which importantly emphasized the systemic nature FMD and the association with aneurysm and dissection in patients with FMD.
Harrison EG Jr, McCormack LJ. Pathologic classification of renal arterial disease in renovascular hypertension. Mayo Clin Proc. 1971;46(3):161–7.
Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129(9):1048–78.
Savard S, Steichen O, Azarine A, Azizi M, Jeunemaitre X, Plouin PF. Association between 2 angiographic subtypes of renal artery fibromuscular dysplasia and clinical characteristics. Circulation. 2012;126(25):3062–9.
Gladstien K, Rushton AR, Kidd KK. Penetrance estimates and recurrence risks for fibromuscular dysplasia. Clin Genet. 1980;17(2):115–6.
Bigazzi R, Bianchi S, Quilici N, Salvadori R, Baldari G. Bilateral fibromuscular dysplasia in identical twins. Am J Kidney Dis. 1998;32(6):E4.
Pannier-Moreau I, Grimbert P, Fiquet-Kempf B, Vuagnat A, Jeunemaitre X, Corvol P, et al. Possible familial origin of multifocal renal artery fibromuscular dysplasia. J Hypertens. 1997;15(12 Pt 2):1797–801.
Petit H, Bouchez B, Destee A, Clarisse J. Familial form of fibromuscular dysplasia of the internal carotid artery. J Neuroradiol. 1983;10(1):15–22.
Rushton AR. The genetics of fibromuscular dysplasia. Arch Intern Med. 1980;140(2):233–6.
Sang CN, Whelton PK, Hamper UM, Connolly M, Kadir S, White RI, et al. Etiologic factors in renovascular fibromuscular dysplasia. A case-control study. Hypertension. 1989;14(5):472–9.
Savard S, Azarine A, Jeunemaitre X, Azizi M, Plouin PF, Steichen O. Association of smoking with phenotype at diagnosis and vascular interventions in patients with renal artery fibromuscular dysplasia. Hypertension. 2013;61(6):1227–32.
Stanley JC, Gewertz BL, Bove EL, Sottiurai V, Fry WJ. Arterial fibrodysplasia. Histopathologic character and current etiologic concepts. Arch Surg. 1975;110(5):561–6.
Ganesh SK, Morissette R, Xu Z, Schoenhoff F, Griswold BF, Yang J, et al. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-β expression and connective tissue features. FASEB J. 2014; doi:10.1096/fj.14-251207.
Frick MP, Goldberg ME. Uro- and angiographic findings in a “normal” population: screening of 151 symptom-free potential transplant donors for renal disease. AJR Am J Roentgenol. 1980;134(3):503–5.
Cragg A, Smith T, Thompson B, Maroney T, Stanson A, Shaw G, et al. Incidental fibromuscular dysplasia in potential renal donors: long-term clinical follow-up. Radiology. 1989;172(1):145–7.
Blondin D, Lanzman R, Schellhammer F, Oels M, Grotemeyer D, Baldus S, et al. Fibromuscular dysplasia in living renal donors: still a challenge to computed tomographic angiography. Eur J Radiol. 2010;75(1):67–71.
Andreoni KA, Weeks SM, Gerber DA, Fair JH, Mauro MA, McCoy L, et al. Incidence of donor renal fibromuscular dysplasia: does it justify routine angiography? Transplantation. 2002;73(7):1112–6.
Lorenz EC, Vrtiska TJ, Lieske JC, Dillon JJ, Stegall MD, Li X, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol. 2010;5(3):431–8.
Neymark E, LaBerge JM, Hirose R, Melzer JS, Kerlan RK Jr, Wilson MW, et al. Arteriographic detection of renovascular disease in potential renal donors: incidence and effect on donor surgery. Radiology. 2000;214(3):755–60.
Spring DB, Satvatierra O Jr, Palubinskas AJ, Amend WJ Jr, Vincenti FG, Feduska NJ. Results and significance of angiography in potential kidney donors 1. Radiology. 1979;133(1):45–7.
Touze E, Oppenheim C, Trystram D, Nokam G, Pasquini M, Alamowitch S, et al. Fibromuscular dysplasia of cervical and intracranial arteries. Int J Stroke. 2010;5(4):296–305.
Shivapour DM, Erwin P, Kim E. Epidemiology of fibromuscular dysplasia: a review of the literature. Vasc Med. 2016;21(4):376–81.
Kadian-Dodov D, Gornik H, Gu X, Froehlich J, Bacharach JM, Gray B, et al. Aneurysm and dissection in fibromuscular dysplasia: findings from the United States registry for FMD. J Am Coll Cardiol. 2014;63 (12_S).
Kadian-Dodov D, Gornik HL, Gu X, Froehlich J, Bacharach JM, Chi YW, et al. Dissection and aneurysm in patients with fibromuscular dysplasia: findings from the U.S. Registry for FMD. J Am Coll Cardiol. 2016;68(2):176–85.
Kim ES, Olin JW, Froehlich JB, Gu X, Bacharach JM, Gray BH, et al. Clinical manifestations of fibromuscular dysplasia vary by patient sex: a report of the United States registry for fibromuscular dysplasia. J Am Coll Cardiol. 2013;62(21):2026–8.
Giacoppo D, Capodanno D, Dangas G, Tamburino C. Spontaneous coronary artery dissection. Int J Cardiol. 2014;175(1):8-20. https://doi.org/10.1016/j.ijcard.2014.04.178.
Alfonso F, Paulo M, Lennie V, Das-Neves B, Echavarría-Pinto M. Fibromuscular dysplasia and spontaneous coronary artery dissection: coincidental association or causality? J Am Coll Cardiol Intv. 2013;6(6):638.
Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014; doi:10.1161/CIRCINTERVENTIONS.114.001760.
Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. J Am Coll Cardiol Intv. 2013;6(1):44–52.
Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management and prognosis of spontaneous coronary artery dissection. Circulation. 2012; doi:10.1161/CIRCULATIONAHA.112.105718.
O’Connor S, Gornik HL, Froehlich JB, Gu X, Gray BH, Mace PD, et al. Smoking and adverse outcomes in fibromuscular dysplasia: U.S. Registry Report. J Am Coll Cardiol. 2016;67(14):1750–1.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S1–45.
Tanemoto M, Takase K, Yamada T, Satoh A, Abe T, Ito S. Dilation of renal artery stenosis after administration of losartan. Hyperten Res. 2007;30(10):999–1002.
Mazza A, Cuppini S, Zamboni S, Schiavon L, Zattoni L, Viale A, et al. Does treatment with olmesartan improve arterial stenoses due to fibromuscular dysplasia? Hypertens Res. 2009;32(10):927–9.
Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124(4):489–532.
Provenzale JM. Dissection of the internal carotid and vertebral arteries: imaging features. AJR Am J Roentgenol. 1995;165(5):1099–104.
Lefebvre P, Cornez N, Quintart C, Motte S, Wautrecht JC. Spontaneous dissection of the internal carotid artery: apropos of a case. Rev Med Brux. 1996;17(5):342–5.
Perez Errazquin F, Gil Peralta A, Garzon FJ, Salinas E, Franco E. Familial internal carotid dissection. Neurologia. 1998;13(5):247–9.
Andre-Sereys P, Petit E, Benrabah R, Abanouh A, Rancurel G, Haut J. Spontaneous dissection of the internal carotid artery in ophthalmological milieu. Apropos of 10 cases. J Fr Ophtalmol. 1996;19(4):259–64.
Sperling W, Kolominsky P, Pfau M, Huk WJ, Stefan H. Dissection of the carotid artery and vertebral artery—diagnosis and therapy. Fortschr Neurol Psychiatr. 1996;64(4):153–60.
Desfontaines P, Despland PA. Dissection of the internal carotid artery: aetiology, symptomatology, clinical and neurosonological follow-up, and treatment in 60 consecutive cases. Acta Neurol Belg. 1995;95(4):226–34.
Nelson EE. Internal carotid artery dissection associated with scuba diving. Ann Emerg Med. 1995;25(1):103–6.
Kumar SD, Kumar V, Kaye W. Bilateral internal carotid artery dissection from vomiting. Am J Emerg Med. 1998;16(7):669–70.
Brandt T, Hausser I, Orberk E, Grau A, Hartschuh W, Anton-Lamprecht I, et al. Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections. Ann Neurol. 1998;44(2):281–5.
van den Berg JS, Limburg M, Kappelle LJ, Pals G, Arwert F, Westerveld A. The role of type III collagen in spontaneous cervical arterial dissections. Ann Neurol. 1998;43(4):494–8.
Mayer SA, Rubin BS, Starman BJ, Byers PH. Spontaneous multivessel cervical artery dissection in a patient with a substitution of alanine for glycine (G13A) in the alpha 1 (I) chain of type I collagen. Neurology. 1996;47(2):552–6.
Peters M, Bohl J, Thomke F, Kallen KJ, Mahlzahn K, Wandel E, et al. Dissection of the internal carotid artery after chiropractic manipulation of the neck. Neurology. 1995;45(12):2284–6.
Boukobza M, Ast G, Reizine D, Merland JJ. Internal carotid artery dissection causes hypoglossal nerve palsy: CT, MRI, and angiographic findings. J Neuroimaging. 1998;8(4):244–6.
Grau AJ, Brandt T, Forsting M, Winter R, Hacke W. Infection-associated cervical artery dissection. Three cases. Stroke. 1997;28(2):453–5.
Thal DR, Schober R, Schlote W. Carotid artery dissection in a young adult: cystic medial necrosis associated with an increased elastase content. Clin Neuropathol. 1997;16(4):180–4.
Patel H, Smith RR, Garg BP. Spontaneous extracranial carotid artery dissection in children. Pediatr Neurol. 1995;13(1):55–60.
Nwokolo N, Bateman DE. Stroke after a visit to the hairdresser. Lancet. 1997;350(9081):866.
Mercier B, Manai R, Cayre-Castel M, Samson Y, Rancurel G. Internal carotid artery dissection following bronchoscopy. J Neurol. 1996;243(4):368–9.
Schievink WI, Mokri B, Whisnant JP. Internal carotid artery dissection in a community. Rochester, Minnesota, 1987–1992. Stroke. 1993;24(11):1678–80.
Giroud M, Fayolle H, Andre N, Dumas R, Becker F, Martin D, et al. Incidence of internal carotid artery dissection in the community of Dijon. J Neurol Neurosurg Psychiatry. 1994;57(11):1443.
Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393–7.
Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947.
Cadiss trial investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361–7.
Hering D, Piper C, Hohmann C, Schultheiss HP, Horstkotte D. Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection. Z Kardiol. 1998;87(12):961–70.
Saw J, Aymong E, Mancini GB, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30(7):814–9.
Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35(2):250–4.
• Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7(5):645–55. This study was one of the first to describe the strong association between SCAD and FMD and to highlight the need to investigate for vascular disease in patients with SCAD.
Ito H, Taylor L, Bowman M, Fry ET, Hermiller JB, Van Tassel JW. Presentation and therapy of spontaneous coronary artery dissection and comparisons of postpartum versus nonpostpartum cases. Am J Cardiol. 2011;107(11):1590–6.
Habakuk O, Goland S, Mehra A, Elkayam U. Pregnancy and the Risk of Spontaneous Coronary Artery Dissection. Circulation: Cardiovascular Interventions. 2017;10:e004941.
Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016;68(3):297–312.
Wigley FM, Flavahan NA. Raynaud’s phenomenon. N Engl J Med. 2016;375(6):556–65.
Roquelaure Y, Ha C, Le Manac’h AP, Bodin J, Bodere A, Bosseau C, et al. Risk factors for Raynaud’s phenomenon in the workforce. Arthritis Care Res (Hoboken). 2012;64(6):898–904.
Freedman RR, Mayes MD. Familial aggregation of primary Raynaud’s disease. Arthritis Rheum. 1996;39(7):1189–91.
Chikura B, Moore T, Manning J, Vail A, Herrick AL. Thumb involvement in Raynaud’s phenomenon as an indicator of underlying connective tissue disease. J Rheumatol. 2010;37(4):783–6.
Plissonneau Duquene P, Pistorius MA, Pottier P, Aymard B, Planchon B. Cold climate could be an etiologic factor involved in Raynaud’s phenomenon physiopathology. Epidemiological investigation from 954 consultations in general practic. Int Angiol. 2015;34(5):467–74.
Tran C, McEwen G, Fraga GR. Chilblain-like leukaemia cutis. BMJ Case Rep. 2016; doi:10.1136/bcr-2016-214838.
Gunther C, Berndt N, Wolf C, Lee-Kirsch MA. Familial chilblain lupus due to a novel mutation in the exonuclease III domain of 3′ repair exonuclease 1 (TREX1). JAMA Dermatol. 2015;151(4):426–31.
Takci Z, Vahaboglu G, Eksioglu H. Epidemiological patterns of perniosis, and its association with systemic disorder. Clin Exp Dermatol. 2012;37(8):844–9.
Cribier B, Djeridi N, Peltre B, Grosshans E. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45(6):924–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Women’s Health
Rights and permissions
About this article
Cite this article
Joseph, L., Kim, E.S.H. Non-Atherosclerotic Vascular Disease in Women. Curr Treat Options Cardio Med 19, 78 (2017). https://doi.org/10.1007/s11936-017-0579-6
Published:
DOI: https://doi.org/10.1007/s11936-017-0579-6