Abstract
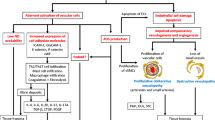
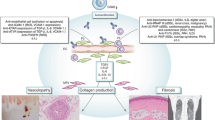
Systemic sclerosis (scleroderma [SSc]) is a multifactorial disease characterized by inflammation, extensive and progressive fibrosis, and multiple vasculopathies. The vascular manifestations can be seen early in the pathogenesis of the disease and include malformed capillaries, Raynaud’s phenomenon, and digital ulcers. As the disease progresses, the vasculopathy proceeds to significant clinical manifestations, including renal crisis and pulmonary arterial hypertension. Moreover, later stages of the disease are marked by increasingly avascular areas. Despite the obliteration of microvascular structures, compensatory vasculogenesis and angiogenesis do not occur normally. This is in spite of a general increase in many potent angiogenic factors. Recent studies are beginning to examine this paradox and subsequent paucity of an angiogenic response in SSc. In this review, we discuss these findings and examine the role that chemokine and growth factor receptors, proteases, adhesion molecules, and transcription factors play in the dysregulation of angiogenesis in SSc.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Gabrielli A, Avvedimento EV, Krieg T: Scleroderma. N Engl J Med 2009, 360(19):1989–2003. This is an excellent recent review on the pathogenesis of SSc.
Maricq HR. Wide-field capillary microscopy. Arthritis Rheum. 1981;24(9):1159–65.
Lambova SN, Muller-Ladner U. Capillaroscopic pattern in systemic sclerosis—an association with dynamics of processes of angio- and vasculogenesis. Microvasc Res. 2010;80(3):534–9.
Hachulla E, Clerson P, Launay D, et al. Natural history of ischemic digital ulcers in systemic sclerosis: single-center retrospective longitudinal study. J Rheumatol. 2007;34(12):2423–30.
Lambova S, Muller-Ladner U. Pulmonary arterial hypertension in systemic sclerosis. Autoimmun Rev. 2010;9(11):761–70.
Campo A, Mathai SC, Le Pavec J, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182(2):252–60.
Denton CP, Lapadula G, Mouthon L, Muller-Ladner U. Renal complications and scleroderma renal crisis. Rheumatology (Oxford). 2009;48 Suppl 3:iii32–5.
Au K, Singh MK, Bodukam V et al.: Atherosclerosis in systemic sclerosis—a systematic review and meta analysis. Arthritis Rheum 2011, Epub ahead of print.
Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–8.
Manetti M, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M. Mechanisms in the loss of capillaries in systemic sclerosis: angiogenesis versus vasculogenesis. J Cell Mol Med. 2010;14(6A):1241–54.
Kuwana M, Okazaki Y, Yasuoka H, et al. Defective vasculogenesis in systemic sclerosis. Lancet. 2004;364(9434):603–10.
Allanore Y, Batteux F, Avouac J, et al. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007;25(1):60–6.
Avouac J, Juin F, Wipff J, et al. Circulating endothelial progenitor cells in systemic sclerosis: association with disease severity. Ann Rheum Dis. 2008;67(10):1455–60.
Del Papa N, Quirici N, Soligo D, et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006;54(8):2605–15.
Distler JH, Allanore Y, Avouac J, et al. EULAR Scleroderma Trials and Research group statement and recommendations on endothelial precursor cells. Ann Rheum Dis. 2009;68(2):163–8.
Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–53.
Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45(4):530–44.
•• Yamaguchi Y, Okazaki Y, Seta N et al.: Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis. Arthritis Res Ther 2010, 12(6):R205. This was the first study to describe monocytic EPCs in SSc. The report further demonstrates that vasculogenesis is impaired in SSc and shows how monocytic EPCs may play a role in angiogenesis.
Kaminski MJ, Majewski S, Jablonska S, Pawinska M. Lowered angiogeneic capability of peripheral blood lymphocytes in progressive systemic sclerosis (scleroderma). J Invest Dermatol. 1984;82(3):239–43.
Majewski S, Skopinska-Rozewska E, Jablonska S, et al. Modulatory effect of sera from scleroderma patients on lymphocyte-induced angiogenesis. Arthritis Rheum. 1985;28(10):1133–9.
Koch AE, Polverini PJ, Leibovich SJ. Induction of neovascularization by activated human monocytes. J Leukoc Biol. 1986;39:233–8.
Marczak M, Majewski S, Skopinska-Rozewska E, et al. Enhanced angiogenic capability of monocyte-enriched mononuclear cell suspensions from patients with systemic scleroderma. J Invest Dermatol. 1986;86(4):355–8.
Kahaleh MB, DeLustro F, Bock W, LeRoy EC. Human monocyte modulation of endothelial cells and fibroblast growth: possible mechanism for fibrosis. Clin Immunol Immunopathol. 1986;39(2):242–55.
Liakouli V, Cipriani P, Marrelli A et al.: Angiogenic cytokines and growth factors in systemic sclerosis. Autoimmun Rev 2011.
•• Margheri F, Serrati S, Lapucci A et al.: Modulation of the angiogenic phenotype of normal and systemic sclerosis endothelial cells by gain-loss of function of pentraxin 3 and matrix metalloproteinase 12. Arthritis Rheum 2010, 62(8):2488–2498. This excellent study expanded on the observation that MMP-12 and pentraxin 3 are overexpressed in SSc by performing an in vitro analysis of their role in angiogenesis. The authors showed that overexpressing both molecules in healthy ECs resulted in an inhibition of angiogenesis. They also found that SSc ECs do not mount an angiogenic response to bFGF or VEGF.
Mackiewicz Z, Sukura A, Povilenaite D, et al. Increased but imbalanced expression of VEGF and its receptors has no positive effect on angiogenesis in systemic sclerosis skin. Clin Exp Rheumatol. 2002;20(5):641–6.
Distler O, Distler JH, Scheid A, et al. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res. 2004;95(1):109–16.
Davies CA, Jeziorska M, Freemont AJ, Herrick AL. The differential expression of VEGF, VEGFR-2, and GLUT-1 proteins in disease subtypes of systemic sclerosis. Hum Pathol. 2006;37(2):190–7.
Carulli MT, Ong VH, Ponticos M, et al. Chemokine receptor CCR2 expression by systemic sclerosis fibroblasts: evidence for autocrine regulation of myofibroblast differentiation. Arthritis Rheum. 2005;52(12):3772–82.
• Rabquer BJ, Tsou P, Hou Y et al.: Dysregulated expression of MIG/CXCL9, IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthritis Res Ther 2011, 13(1):R18. This study provides further evidence of the importance of determining the expression of both angiogenic mediators and their receptors. It demonstrated that while antiangiogenic chemokines are elevated in SSc, their receptor is downregulated, thus limiting their impact.
Cipriani P, Franca Milia A, Liakouli V, et al. Differential expression of stromal cell-derived factor 1 and its receptor CXCR4 in the skin and endothelial cells of systemic sclerosis patients: pathogenetic implications. Arthritis Rheum. 2006;54(9):3022–33.
Rabquer BJ, Boychev G, Ruth JH, et al. Soluble junctional adhesion molecule-A promotes angiogenesis in rheumatoid arthritis. Arthritis Rheum. 2010;62:S590.
Hou Y, Rabquer BJ, Gerber ML, et al. Junctional adhesion molecule-A is abnormally expressed in diffuse cutaneous systemic sclerosis skin and mediates myeloid cell adhesion. Ann Rheum Dis. 2009;69(1):249–54.
Naik MU, Mousa SA, Parkos CA, Naik UP. Signaling through JAM-1 and alphavbeta3 is required for the angiogenic action of bFGF: dissociation of the JAM-1 and alphavbeta3 complex. Blood. 2003;102(6):2108–14.
D’Alessio S, Fibbi G, Cinelli M, et al. Matrix metalloproteinase 12-dependent cleavage of urokinase receptor in systemic sclerosis microvascular endothelial cells results in impaired angiogenesis. Arthritis Rheum. 2004;50(10):3275–85.
Giusti B, Fibbi G, Margheri F, et al. A model of anti-angiogenesis: differential transcriptosome profiling of microvascular endothelial cells from diffuse systemic sclerosis patients. Arthritis Res Ther. 2006;8(4):R115.
Serrati S, Cinelli M, Margheri F, et al. Systemic sclerosis fibroblasts inhibit in vitro angiogenesis by MMP-12-dependent cleavage of the endothelial cell urokinase receptor. J Pathol. 2006;210(2):240–8.
Manetti M, Allanore Y, Revillod L, et al. A genetic variation located in the promoter region of the UPAR (CD87) gene is associated with the vascular complications of systemic sclerosis. Arthritis Rheum. 2011;63(1):247–56.
Dean RA, Cox JH, Bellac CL, et al. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR + CXC chemokines and generates CCL2, −7, −8, and −13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112(8):3455–64.
Giusti B, Serrati S, Margheri F, et al. The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis. Arthritis Rheum. 2005;52(11):3618–28.
Margheri F, Manetti M, Serrati S, et al. Domain 1 of the urokinase-type plasminogen activator receptor is required for its morphologic and functional, beta2 integrin-mediated connection with actin cytoskeleton in human microvascular endothelial cells: failure of association in systemic sclerosis endothelial cells. Arthritis Rheum. 2006;54(12):3926–38.
Wagner EF. Bone development and inflammatory disease is regulated by AP-1 (Fos/Jun). Ann Rheum Dis. 2010;69 Suppl 1:i86–8.
Eferl R, Zenz R, Theussl HC, Wagner EF. Simultaneous generation of fra-2 conditional and fra-2 knock-out mice. Genesis. 2007;45(7):447–51.
Eferl R, Hasselblatt P, Rath M, et al. Development of pulmonary fibrosis through a pathway involving the transcription factor Fra-2/AP-1. Proc Natl Acad Sci USA. 2008;105(30):10525–30.
•• Maurer B, Busch N, Jungel A et al.: Transcription factor fos-related antigen-2 induces progressive peripheral vasculopathy in mice closely resembling human systemic sclerosis. Circulation 2009, 120(23):2367–2376. This study showed that mice overexpressing Fra-2 show both fibrotic and vascular characteristics of SSc. In addition, the study showed that Fra-2 is overexpressed in SSc ECs. The authors also demonstrated that blocking Fra-2 in ECs enhances VEGF-mediated angiogenesis in vitro. In all, this study established Fra-2 as an angiogenic transcription factor and demonstrated that Fra-2 transgenic mice may be an excellent model of SSc.
Reich N, Maurer B, Akhmetshina A, et al. The transcription factor Fra-2 regulates the production of extracellular matrix in systemic sclerosis. Arthritis Rheum. 2010;62(1):280–90.
Spyropoulos DD, Pharr PN, Lavenburg KR, et al. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20(15):5643–52.
Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18(16):1234–40.
Kubo M, Czuwara-Ladykowska J, Moussa O, et al. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am J Pathol. 2003;163(2):571–81.
•• Asano Y, Stawski L, Hant F et al.: Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am J Pathol 2010, 176(4):1983–1998. This novel study expanded on the finding that Fli1 is decreased in SSc ECs by generating mice with Fli1 conditionally knocked out on ECs. The mice exhibited an “SSc-like” phenotype and implicated a role for Fli1 in the vascular pathology of SSc. In addition, the murine model has the potential to serve as a model of SSc vascular disease.
Acknowledgments
This work was supported by the National Institutes of Health (grant no. HL094017 to Dr. Rabquer), the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, the Frederick G.L. Huetwell and William D. Robinson, MD, Professorship in Rheumatology, and by the Scleroderma Foundation (Mark Flapan Award).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabquer, B.J., Koch, A.E. Angiogenesis and Vasculopathy in Systemic Sclerosis: Evolving Concepts. Curr Rheumatol Rep 14, 56–63 (2012). https://doi.org/10.1007/s11926-011-0219-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-011-0219-1