Abstract
Purpose of Review
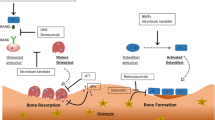
The skeletal system provides an important role to support body structure and protect organs. The complexity of its architecture and components makes it challenging to deliver the right amount of the drug into bone regions, particularly avascular cartilage lesions. In this review, we describe the recent advance of bone-targeting methods using bisphosphonates, polymeric oligopeptides, and nanoparticles on osteoporosis and rare skeletal diseases.
Recent Findings
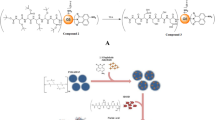
Hydroxyapatite (HA), a calcium phosphate with the formula Ca10(PO4)6(OH)2, is a primary matrix of bone mineral that includes a high concentration of positively charged calcium ion and is found only in the bone. This unique feature makes HA a general targeting moiety to the entire skeletal system. We have applied bone-targeting strategy using acidic amino acid oligopeptides into lysosomal enzymes, demonstrating the effects of bone-targeting enzyme replacement therapy and gene therapy on bone and cartilage lesions in inherited skeletal disorders. Virus or no-virus gene therapy using techniques of engineered capsid or nanomedicine has been studied preclinically for skeletal diseases.
Summary
Efficient drug delivery into bone lesions remains an unmet challenge in clinical practice. Bone-targeting therapies based on gene transfer can be potential as new candidates for skeletal diseases.



Similar content being viewed by others
Abbreviations
- AAV:
-
Adeno-associated virus
- ADL:
-
Activity of daily living
- ALN:
-
Alendronate
- Asp:
-
Aspartic acid
- BMD:
-
Bone mineral density
- BPs:
-
Bisphosphonates
- C6S:
-
Chondroitin-6-sulfate
- D8:
-
Aspartic acid octapeptide
- E2:
-
Estradiol
- ERT:
-
Enzyme replacement therapy
- GAG:
-
Glycosaminoglycans
- GALNS:
-
N-Acetylgalactosamine 6-sulfate sulfatase
- Glu:
-
Glutamic acid
- HA:
-
Hydroxyapatite
- HPP:
-
Hypophosphatasia
- HS:
-
Heparan sulfate
- HSCT:
-
Hematopoietic stem cell transplantation
- IV:
-
Intravenous
- KS:
-
Keratan sulfate
- miRNA:
-
MicroRNA
- MPS:
-
Mucopolysaccharidosis
- N-BPs:
-
Nitrogen-containing BPs
- NLC:
-
Nanostructured lipid carriers
- NPs:
-
Nanoparticles
- OVX:
-
Ovariectomized
- PEG:
-
Polyethylene glycol
- PLGA:
-
Poly (d,l-lactic-co-glycolic acid)
- siRNA:
-
Small interfering RNA
References
Farrell KB, Karpeisky A, Thamm DH, Zinnen S. Bisphosphonate conjugation for bone specific drug targeting [Internet]. Bone Rep. 2018:47–60 Elsevier Inc; [cited 2020 Apr 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29992180.
Riggs BL, Melton LJ. Involutional osteoporosis. N Engl J Med. 1986:1676–86.
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–6 [Internet]. John Wiley and Sons Inc. [cited 2020 Apr 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24771492.
Stapleton M, Sawamoto K, Almeciga-Diaz CJ, Mackenzie WG, Mason RW, Orii T, et al. Development of bone targeting drugs. Int J Mol Sci. 2017;18. Department of Biological Sciences, University of Delaware, Newark, DE 19716, USA. mstaple@udel.edu.; Nemours/Alfred I. duPont Hospital for Children, Wilmington, DE 19803, USA. mstaple@udel.edu.; Nemours/Alfred I. duPont Hospital for Children, Wilmington. https://doi.org/10.3390/ijms18071345.
Carbone EJ, Rajpura K, Allen BN, Cheng E, Ulery BD, Lo KWH. Osteotropic nanoscale drug delivery systems based on small molecule bone-targeting moieties. Nanomed Nanotechnol Biol Med. 2017:37–47 Elsevier Inc.
Wang D, Miller SC, Kopečková P, Kopeček J. Bone-targeting macromolecular therapeutics. Adv Drug Deliv Rev. 2005:1049–76.
Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008:S131 American Society of Nephrology.
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019:908–31 Elsevier B.V.
Russell RGG. Bisphosphonates: the first 40 years. Bone. 2011:2–19.
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38:617–27.
Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008:1032–45 Elsevier Ltd.
Doschak MR, Kucharski CM, Wright JEI, Zernicke RF, Uludag H. Improved bone delivery of osteoprotegerin by bisphosphonate conjugation in a rat model of osteoarthritis. Mol Pharm. 2009;6:634–40 [Internet]. [cited 2020 Apr 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19718808.
Zheng Y, Nishikawa M, Ikemura M, Yamashita F, Hashida M. Development of bone-targeted catalase derivatives for inhibition of bone metastasis of tumor cells in mice. J Pharm Sci. 2012;101:552–7 [Internet]. John Wiley and Sons Inc. [cited 2020 Apr 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21953593.
Katsumi H, Sano JI, Nishikawa M, Hanzawa K, Sakane T, Yamamoto A. Molecular design of bisphosphonate-modified proteins for efficient bone targeting in vivo. PLoS One. 2015;10 Public Library of Science.
Butler WT. The nature and significance of osteopontin. Connect Tissue Res. 1989;23:123–36 Informa Healthcare.
Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986;83:8819–23.
Oldberg A, Franzen A, Heinegard D. The primary structure of a cell-binding bone sialoprotein. J Biol Chem. 1988;263:19430–2.
Fujisawa R, Wada Y, Nodasaka Y, Kuboki Y. Acidic amino acid-rich sequences as binding sites of osteonectin to hydroxyapatite crystals. Biochim Biophys Acta Protein Struct Mol Enzymol. 1996;1292:53–60 Elsevier B.V.
Sekido T, Sakura N, Higashi Y, Miya K, Nitta Y, Nomura M, et al. Novel drug delivery system to bone using acidic oligopeptide: pharmacokinetic characteristics and pharmacological potential. J Drug Target. 2001;9:111–21. [Internet]. Taylor & Francis Available from. https://doi.org/10.3109/10611860108997922.
Low SA, Kopecek J. Targeting polymer therapeutics to bone. Adv Drug Deliv Rev. 2012;64:1189–204 Department of Bioengineering, University of Utah, Salt Lake City, UT 84112, USA.: Elsevier B.V.
Vinay R, KusumDevi V. Potential of targeted drug delivery system for the treatment of bone metastasis. Drug Deliv. 2016;23:21–9. [Internet]. Taylor & Francis; Available from. https://doi.org/10.3109/10717544.2014.913325.
Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat Med. 2012;18:307–14 Musculoskeletal Research Laboratory, Department of Orthopaedics & Traumatology, The Chinese University of Hong Kong, Hong Kong, China.
Sipila S, Tormakangas T, Sillanpaa E, Aukee P, Kujala UM, Kovanen V, et al. Muscle and bone mass in middle-aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020; Gerontology Research Center, Faculty of Sport and Health Sciences, University of Jyvaskyla, Jyvaskyla, Finland.; Gerontology Research Center, Faculty of Sport and Health Sciences, University of Jyvaskyla, Jyvaskyla, Finland.; Gerontology Research Center, : The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Luhmann T, Germershaus O, Groll J, Meinel L. Bone targeting for the treatment of osteoporosis. J Control Release. 2012;161:198–213 Institute for Pharmacy and Food Chemistry, University of Wurzburg, Am Hubland, DE-97074 Wurzburg, Germany.: Elsevier B.V.
Alméciga-Díaz CJ, Montaño AM, Barrera LA, Tomatsu S. Tailoring the AAV2 capsid vector for bone-targeting. Pediatr Res. 2018;84:545–51. [Internet]. Available from. https://doi.org/10.1038/s41390-018-0095-8.
Alméciga-Diaz CJ, Barrera LA. Design and applications of gene therapy vectors for mucopolysaccharidosis in Colombia. Gene Ther. 2020;27:104–7. [Internet] Available from. https://doi.org/10.1038/s41434-019-0086-3.
Ray K. Silencing inhibitors of bone formation. Nat Rev Rheumatol. 2012;8:122. [Internet]. Available from. https://doi.org/10.1038/nrrheum.2012.17.
Alencastre I, Sousa D, Alves C, Leitão L, Neto E, Aguiar P, et al. Delivery of pharmaceutics to bone: nanotechnologies, high-throughput processing and in silico mathematical models. Eur Cell Mater. 2016;31:355–81.
Jiang B, Cao J, Zhao J, He D, Pan J, Li Y, et al. Dual-targeting delivery system for bone cancer: synthesis and preliminary biological evaluation. Drug Deliv. 2012;19:317–26. [Internet]. Taylor & Francis. Available from. https://doi.org/10.3109/10717544.2012.714809.
Li CJ, Liu XZ, Zhang L, Chen LB, Shi X, Wu SJ, et al. Advances in bone-targeted drug delivery systems for neoadjuvant chemotherapy for osteosarcoma. Orthop Surg. 2016;8:105–10 Department of Orthopaedics, School of Medicine, Jinling Hospital, Nanjing University, Nanjing, China.; Department of Orthopaedics, School of Medicine, Jinling Hospital, Nanjing University, Nanjing, China.; Department of Orthopaedics, School of Medicine, J: Chinese Orthopaedic Association and John Wiley & Sons Australia, Ltd.
Aoki K, Alles N, Soysa N, Ohya K. Peptide-based delivery to bone [Internet]. Adv Drug Deliv Rev. 2012:1220–38 [cited 2020 Jul 13]. Available from: https://pubmed.ncbi.nlm.nih.gov/22709649/.
Nguyen TBL, Min YK, Lee B-T. Nanoparticle biphasic calcium phosphate loading on gelatin-pectin scaffold for improved bone regeneration. Tissue Eng Part A. 2015;21:1376–87 [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25602709.
Ma X, Gong N, Zhong L, Sun J, Liang X-J. Future of nanotherapeutics: targeting the cellular sub-organelles. Biomaterials. 2016;97:10–21 [Internet]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0142961216301375.
Parent M, Baradari H, Champion E, Damia C, Viana-Trecant M. Design of calcium phosphate ceramics for drug delivery applications in bone diseases: a review of the parameters affecting the loading and release of the therapeutic substance. J Control Release. 2017;252:1–17 [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28232225.
Melville AJ, Rodríguez-Lorenzo LM, Forsythe JS. Effects of calcination temperature on the drug delivery behaviour of ibuprofen from hydroxyapatite powders. J Mater Sci Mater Med. 2008;19:1187–95 [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17701302.
Matsumoto T, Okazaki M, Inoue M, Yamaguchi S, Kusunose T, Toyonaga T, et al. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials. 2004;25:3807–12 [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15020156.
Lin K, Wu C, Chang J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014;10:4071–102 [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24954909.
Uskoković V, Batarni SS, Schweicher J, King A, Desai TA. Effect of calcium phosphate particle shape and size on their antibacterial and osteogenic activity in the delivery of antibiotics in vitro. ACS Appl Mater Interfaces 2013;5(7):2422–31. https://doi.org/10.1021/am4000694
Feng B, Weng J, Yang BC, Qu SX, Zhang XD. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials. 2003;24:4663–70 [Internet] Available from: https://linkinghub.elsevier.com/retrieve/pii/S0142961203003661.
Chou LYT, Ming K, Chan WCW. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–45 [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20886124.
Kruger CA, Abrahamse H. Utilisation of targeted nanoparticle photosensitiser drug delivery systems for the enhancement of photodynamic therapy [Internet]. Molecules. 2018; MDPI AG. [Cited 2020 Apr 17]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30322132.
Ojea-Jimenez I, Comenge J, Garcia-Fernandez L, Megson Z, Casals E, Puntes V. Engineered inorganic nanoparticles for drug delivery applications. Curr Drug Metab. 2013;14:518–30 Bentham Science Publishers Ltd.
Choi S-W, Kim J-H. Design of surface-modified poly(D,L-lactide-co-glycolide) nanoparticles for targeted drug delivery to bone. J Control Release. 2007;122:24–30.
Ramanlal Chaudhari K, Kumar A, Megraj Khandelwal VK, Ukawala M, Manjappa AS, Mishra AK, et al. Bone metastasis targeting: a novel approach to reach bone using zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J Control Release. 2012;158:470–8 [Internet]. [cited 2020 Apr 17]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22146683.
Salerno M, Cenni E, Fotia C, Avnet S, Granchi D, Castelli F, et al. Bone-targeted doxorubicin-loaded nanoparticles as a tool for the treatment of skeletal metastases. Curr Cancer Drug Targets. 2010;10:649–59 [Internet]. Bentham Science Publishers Ltd. [cited 2020 Apr 17]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20578992.
Pignatello R, Sarpietro MG, Castelli F. Synthesis and biological evaluation of a new polymeric conjugate and nanocarrier with osteotropic properties. J Funct Biomater. 2012;3:79–99 MDPI AG.
Morton SW, Shah NJ, Quadir MA, Deng ZJ, Poon Z, Hammond PT. Osteotropic therapy via targeted layer-by-layer nanoparticles. Adv Healthc Mater. 2014;3:867–75 Wiley-VCH Verlag.
Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S, et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc Natl Acad Sci U S A. 2014;111:10287–92 [Internet]. National Academy of Sciences. [cited 2020 Apr 17]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24982170.
Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21:103–13.
Sowers MFR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–7.
Yokogawa K, Miya K, Sekido T, Higashi Y, Nomura M, Fujisawa R, et al. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology. 2001;142:1228–33 Department of Hospital Pharmacy, School of Medicine, Kanazawa University, Kanazawa 920–8641, Japan.
Ishizaki J, Waki Y, Takahashi-Nishioka T, Yokogawa K, Miyamoto K. Selective drug delivery to bone using acidic oligopeptides. J Bone Miner Metab. 2009;27:1–8. [Internet]. Available from. https://doi.org/10.1007/s00774-008-0004-z.
Yang Y, Aghazadeh-Habashi A, Panahifar A, Wu Y, Bhandari KH, Doschak MR. Bone-targeting parathyroid hormone conjugates outperform unmodified PTH in the anabolic treatment of osteoporosis in rats. Drug Deliv Transl Res. 2017;7:482–96 [Internet]. Springer Verlag. [cited 2020 Apr 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28721611.
Bhandari K, Asghar W, Newa M, Jamali F, Doschak M. Evaluation of bone targeting salmon calcitonin analogues in rats developing osteoporosis and adjuvant arthritis. Curr Drug Deliv. 2015;12:98–107 Bentham Science Publishers Ltd.
Gil L, Han Y, Opas EE, Rodan GA, Ruel R, Seedor JG, et al. Prostaglandin E2-bisphosphonate conjugates: potential agents for treatment of osteoporosis. Bioorg Med Chem. 1999;7:901–19.
Tsushima N, Yabuki M, Harada H, Katsumata T, Kanamaru H, Nakatsuka I, et al. Tissue distribution and pharmacological potential of SM-16896, a novel oestrogen-bisphosphonate hybrid compound*. J Pharm Pharmacol. 2000;52:27–37 Wiley.
Tomatsu S, Montaño AM, Oikawa H, Giugliani R, Harmatz PR, Smith M, et al. Chapter 126 impairment of body growth in mucopolysaccharidoses. 2011.
Melbouci M, Mason RW, Suzuki Y, Fukao T, Orii T, Tomatsu S. Growth impairment in mucopolysaccharidoses. Mol Genet Metab. 2018:1–10 Academic Press Inc.
Tomatsu S, Alméciga-Díaz CJ, Montaño AM, Yabe H, Tanaka A, Dung VC, et al. Therapies for the bone in mucopolysaccharidoses. Mol Genet Metab. 2015:94–109 Academic Press Inc.
Kecskemethy HH, Kubaski F, Harcke HT, Tomatsu S. Bone mineral density in MPS IV A (Morquio syndrome type A). Mol Genet Metab. 2016;117:144–9 Academic Press Inc.
Yasuda E, Suzuki Y, Shimada T, Sawamoto K, Mackenzie WG, Theroux MC, et al. Activity of daily living for Morquio A syndrome. Mol Genet Metab. 2016;118:111–22 Academic Press Inc.
Doherty C, Stapleton M, Piechnik M, Mason RW, Mackenzie WG, Yamaguchi S, et al. Effect of enzyme replacement therapy on the growth of patients with Morquio A. J Hum Genet. 2019;64:625–35 Nature Publishing Group.
Yabe H, Tanaka A, Chinen Y, Kato S, Sawamoto K, Yasuda E, et al. Hematopoietic stem cell transplantation for Morquio A syndrome. Mol Genet Metab. 2016;117:84–94 [Internet]. Academic Press Inc.; [cited 2020 Apr 17]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26452513.
Chinen Y, Higa T, Tomatsu S, Suzuki Y, Orii T, Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol Genet Metab Rep. 2014;1:31–41 Elsevier Inc.
Tomatsu S, Montão AM, Dung VC, Ohashi A, Oikawa H, Oguma T, et al. Enhancement of drug delivery: enzyme-replacement therapy for murine morquio a syndrome. Mol Ther. 2010;18:1094–102.
Tomatsu S, Montaño AM, Ohashi A, Gutierrez MA, Oikawa H, Oguma T, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–24. [Internet]. Available from. https://doi.org/10.1093/hmg/ddm353.
Víctor Álvarez J, Bravo SB, García-Vence M, De Castro MJ, Luzardo A, Colón C, et al. Proteomic analysis in morquio a cells treated with immobilized enzymatic replacement therapy on nanostructured lipid systems. Int J Mol Sci. 2019;20 MDPI AG.
Sawamoto K, González JVÁ, Piechnik M, Otero FJ, Couce ML, Suzuki Y, et al. Mucopolysaccharidosis IVA: diagnosis, treatment, and management. Int J Mol Sci. 2020; MDPI AG.
Nishioka T, Tomatsu S, Gutierrez MA, Miyamoto K, Trandafirescu GG, Lopez PLC, et al. Enhancement of drug delivery to bone: characterization of human tissue-nonspecific alkaline phosphatase tagged with an acidic oligopeptide [Internet]. Mol Genet Metab. 2006:244–55 Available from: http://www.sciencedirect.com/science/article/pii/S1096719206000758.
Whyte MP. Hypophosphatasia: an overview for 2017. Bone. 2017;102:15–25 Center for Metabolic Bone Disease and Molecular Research, Shriners Hospital for Children, Division of Bone and Mineral Diseases, Department of Internal Medicine, Washington University School of Medicine at Barnes-Jewish Hospital, St. Louis, Missouri, USA.: Elsevier Inc.
Whyte MP. Hypophosphatasia: enzyme replacement therapy brings new opportunities and new challenges. J Bone Miner Res. 2017;32:667–75. [Internet]. John Wiley & Sons, Ltd. Available from. https://doi.org/10.1002/jbmr.3075.
Mornet E. Genetics of Hypophosphatasia. Clin Rev Bone Miner Metab. 2013;11:71–7. [Internet]. Available from. https://doi.org/10.1007/s12018-013-9140-7.
Castells L, Cassanello P, Muñiz F, De Castro MJ, Couce ML. Neonatal lethal hypophosphatasia A case report and review of literature. Medicine. 2018:97 Lippincott Williams and Wilkins.
Whyte MP, Habib D, Coburn SP, Tecklenburg F, Ryan LM, Fedde K, et al. Failure of hyper-phosphatasemia by intravenous infusion of purified placental alkaline phosphatase (ALP) to correct severe hypophosphatasia: evidence against. J Bone Miner Res. 1992;7.
Whyte MP, Landt M, Ryan LM, Mulivor RA, Henthorn PS, Fedde KN, et al. Alkaline phosphatase: placental and tissue-nonspecific isoenzymes hydrolyze phosphoethanolamine, inorganic pyrophosphate, and pyridoxal 5’-phosphate. Substrate accumulation in carriers of hypophosphatasia corrects during pregnancy. J Clin Invest. 1995;95:1440–5 Metabolic Research Unit, Shriners Hospital for Crippled Children, St. Louis, Missouri 63131, USA.
Whyte MP, Magill HL, Fallon MD, Herrod HG. Infantile hypophosphatasia: normalization of circulating bone alkaline phosphatase activity followed by skeletal remineralization. Evidence for an intact structural gene for tissue nonspecific alkaline phosphatase. J Pediatr United States. 1986;108:82–8.
Mornet E. Hypophosphatasia. Metabolism. 2018;82:142–55 Unite de Genetique Constitutionnelle, Service de Biologie, Centre Hospitalier de Versailles, 177 rue de Versailles, 78150 Le Chesnay, France. Electronic address: emornet@ch-versailles.fr.: Elsevier Inc.
Millan JL, Whyte MP. Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int. 2016;98:398–416 Sanford Children’s Health Research Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, CA, 92037, USA. millan@burnham.org.; Center for Metabolic Bone Disease and Molecular Research, Shriners Hospital for Children, St. Louis, MO, 63110, U.
Nakano C, Kitabatake Y, Takeyari S, Ohata Y, Kubota T, Taketani K, et al. Genetic correction of induced pluripotent stem cells mediated by transcription activator-like effector nucleases targeting ALPL recovers enzyme activity and calcification in vitro. Mol Genet Metab. 2019;127:158–65 [Internet]. Academic Press Inc.; [cited 2020 Apr 20]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31178256.
Matsumoto T, Miyake K, Yamamoto S, Orimo H, Miyake N, Odagaki Y, et al. Rescue of severe infantile hypophosphatasia mice by AAV-mediated sustained expression of soluble alkaline phosphatase. Hum Gene Ther. 2011;22:1355–64 Department of Biochemistry and Molecular Biology, Nippon Medical School, Bunkyo-ku, Tokyo 113–8602, Japan.
Nakamura-Takahashi A, Miyake K, Watanabe A, Hirai Y, Iijima O, Miyake N, et al. Treatment of hypophosphatasia by muscle-directed expression of bone-targeted alkaline phosphatase via self-complementary AAV8 vector [Internet]. Mol Ther Methods Clin Dev. 2016:15059 Available from: http://www.sciencedirect.com/science/article/pii/S2329050116301498.
Yamamoto S, Orimo H, Matsumoto T, Iijima O, Narisawa S, Maeda T, et al. Prolonged survival and phenotypic correction of Akp2−/− hypophosphatasia mice by lentiviral gene therapy. J Bone Miner Res. 2011;26:135–42. [Internet]. John Wiley & Sons, Ltd. Available from. https://doi.org/10.1002/jbmr.201.
Ikeue R, Nakamura-Takahashi A, Nitahara-Kasahara Y, Watanabe A, Muramatsu T, Sato T, et al. Bone-targeted alkaline phosphatase treatment of mandibular bone and teeth in lethal hypophosphatasia via an scAAV8 Vector [Internet]. Mol Ther Methods Clin Dev. 2018:361–70 Available from: http://www.sciencedirect.com/science/article/pii/S2329050118300810.
Nakamura-Takahashi A, Tanase T, Matsunaga S, Shintani S, Abe S, Nitahara-Kasahara Y, et al. High-level expression of alkaline phosphatase by adeno-associated virus vector ameliorates pathological bone structure in a hypophosphatasia mouse model. Calcif Tissue Int. 2020; Department of Pharmacology, Tokyo Dental College, 2-9-18, Kandamisaki-cho, Chiyoda-ku, Tokyo, 101-0061, Japan. atakahashi@tdc.ac.jp.; Tokyo Dental College Research Branding Project, Tokyo Dental College, Tokyo, Japan. atakahashi@tdc.ac.jp.; Department of.
Sun X, Guo Q, Wei W, Robertson S, Yuan Y, Luo X. Current progress on microRNA-based gene delivery in the treatment of osteoporosis and osteoporotic fracture. Dufau ML, editor. Int J Endocrinol. 2019;2019:6782653. [Internet]. Hindawi. Available from. https://doi.org/10.1155/2019/6782653.
Yang Y-S, Xie J, Wang D, Kim J-M, Tai PWL, Gravallese E, et al. Bone-targeting AAV-mediated silencing of Schnurri-3 prevents bone loss in osteoporosis. Nat Commun. 2019;10:2958. [Internet]. Available from. https://doi.org/10.1038/s41467-019-10809-6.
Funding
This work was supported by grants from National MPS Society Research Grant; Austrian MPS Society; The Carol Ann Foundation; Deborah McClellan and Brant Cali Foundation; The Radiant Hope donation; Angelo R. Cali & Mary V. Cali Family Foundation, Inc.; The Vain and Harry Fish Foundation, Inc.; The Bennett Foundation; Jacob Randall Foundation; Help Morquio Foundation; Vice Family; Lubert Family Foundation; Straughan Family; Paidipalli Family; and Nemours Funds. S.T. was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant numbers P20GM103464 and P30GM114736. CJAD was supported by the Ministry of Science, Technology and Innovation (Contract 120380763212—PPTA # 8352).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Regenerative Biology and Medicine in Osteoporosis
Kazuki Sawamoto, J. Víctor Álvarez1, and Angélica María Herreño are joint first authors
Rights and permissions
About this article
Cite this article
Sawamoto, K., Álvarez, J.V., Herreño, A.M. et al. Bone-Specific Drug Delivery for Osteoporosis and Rare Skeletal Disorders. Curr Osteoporos Rep 18, 515–525 (2020). https://doi.org/10.1007/s11914-020-00620-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00620-4