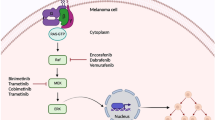
Abstract
The development of targeted therapies has ushered in a new era in the management of melanoma. Inhibitors of the RAS-RAF-MEK-ERK pathway have taken the center stage with development at a rapid pace. Vemurafenib was recently approved by regulatory agencies, and other agents (e.g. dabrafenib) are in various stages of clinical testing. These agents are producing remarkable results for patients, but are also presenting new challenges. Clinical toxicities and drug resistance are topmost issues. Some of the most common and vivid representations of adverse events to these agents are the dermatologic manifestations. Published trials and initial observations reflect a toxicity profile (e.g. squamous cell carcinomas/keratoacanthomas, maculopapular rashes, hyperkeratosis) that is distinct from cutaneous toxicities from EGFR and mTOR inhibitors (acneiform rash, paronychia, xerosis). Their management extends beyond conservative treatment and includes specific physical and surgical treatment modalities, skill sets unique to dermatologists. All these pose significant challenges to clinicians, and sound knowledge of such toxicities and their management will likely result in improved patient outcomes and quality of life. In this manuscript, we provide an overview of the emerging scientific literature on dermatological adverse events arising out of BRAF inhibition.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Downward J. Targeting RAS, signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22.
Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta. 2007;1773(8):1161–76.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54.
Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62(23):6997–7000.
Weber A, Langhanki L, Sommerer F, Markwarth A, Wittekind C, Tannapfel A. Mutations of the BRAF gene in squamous cell carcinoma of the head and neck. Oncogene. 2003;22(30):4757–9.
Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70(13):5518–27.
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47.
Ribas A, Flaherty KT. BRAF targeted therapy changes the treatment paradigm in melanoma. Nat Rev Clin Oncol. 2011;8(7):426–33.
• Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. Elaborate study results can be found on the US National Institutes of Health Clinical trials website: http://clinicaltrials.gov/ct2/show/results/NCT01006980.
Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464(7287):431–5.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–30.
Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–21.
FDA label: Zelboraf (Vemurafenib). San Francisco, CA: Genetech USA Inc. 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202429s000lbl.pdf. Accessed 2 Dec 2012.
FDA label: Nexavar (Sorafenib). Wayne, NJ: Bayer Healthcare Pharmaceuticals Inc. 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021923s011lbl.pdf. Accessed 10 Jan 2013.
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95(5):581–6.
Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7(10):3129–40.
• Arnault JP, Mateus C, Escudier B, Tomasic G, Wechsler J, Hollville E, et al. Skin tumors induced by sorafenib; paradoxic RAS-RAF pathway activation and oncogenic mutations of HRAS, TP53, and TGFBR1. Clin Cancer Res. 2012;18(1):263–72. Mutational analysis of Sorafenib-induced cutaneous lesions induced by Sorafenib. This paper suggests that Sorafenib activates MAPK pathway in normal skin through BRAF-CRAF dimerization, which leads to CRAF activation and eventually keratinocyte proliferation.
Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–19.
• Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–14. This Phase II trial independently confirmed the antitumor activity of vemurafenib in previously treated metastatic melanoma patients, and reported rapid response rates (> 50 %) and impressive overall disease free survival (16 months). Elaborate study results can be found on the US National Institutes of Health Clinical trials website: http://clinicaltrials.gov/ct2/show/results/NCT00949702 .
Lacouture ME, Duvic M, Hauschild A, Prieto VG, Robert C, Schadendorf D et al. Analysis of Dermatologic Events in Vemurafenib-Treated Melanoma Patients. Oncologist. 2013 [In Press]
• Chu EY, Wanat KA, Miller CJ, Amaravadi RK, Fecher LA, Brose MS, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: A clinicopathologic study. J Am Acad Dermatol. 2012;67(6):1265–72. Single institution clinicopathologic study that describes several cutaneous side effects to vemurafenib and dabrafenib.
Robert C, Arnault JP, Mateus C. RAF inhibition and induction of cutaneous squamous cell carcinoma. Curr Opin Oncol. 2011;23(2):177–82.
Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7(1):20–3.
• Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–65. Phase III trial of dabrafenib in patients with V600E-mutated metastatic melanoma resulted in improved progression-free survival (5.1 months) compared with dacarbazine (2.7 months). Most common dermatological adverse events from dabrafenib were hyperkeratosis, papillomas, KAs, SCCs, and, less commonly, BCCs and new primary malignant melanomas.
Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–95.
Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901.
• Anforth RM, Blumetti TC, Kefford RF, Sharma R, Scolyer RA, Kossard S, et al. Cutaneous manifestations of dabrafenib (GSK2118436): a selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br J Dermatol. 2012;167(5):1153–60. Systematic prospective dermatological review of patients enrolled in a phase I/II trial of BRAF inhibitor dabrafenib for treatment of metastatic melanoma. The authors concluded that highly oncogenic HPV infection is not responsible for SCCs and verrucal keratoses.
Mattei PL, Alora-Palli MB, Kraft S, Lawrence DP, Flaherty KT, Kimball AB. Cutaneous effects of BRAF inhibitor therapy: a case series. Ann Oncol. 2012. [Epub Ahead of Print]
Williams VL, Cohen PR, Stewart DJ. Sorafenib-induced premalignant and malignant skin lesions. Int J Dermatol. 2011;50(4):396–402.
Kong HH, Turner ML. Array of cutaneous adverse effects associated with sorafenib. J Am Acad Dermatol. 2009;61(2):360–1.
Robert C, Mateus C, Spatz A, Wechsler J, Escudier B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60(2):299–305.
Arnault JP, Wechsler J, Escudier B, Spatz A, Tomasic G, Sibaud V, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27(23):e59–61.
Huang V, Hepper D, Anadkat M, Cornelius L. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148(5):628–33.
Lacouture ME, Desai A, Soltani K, Petronic-Rosic V, Laumann AE, Ratain MJ, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31(6):783–5.
Ribas A, Kim KB, Schuchter LM, Gonzalez R, Pavlick AC, Weber JS, et al. BRIM-2: an open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAFV600E mutation positive melanoma. J Clin Oncol. 2011;29(Suppl). Abstract 8509.
Larkin JM, Queirolo P, Arance AM, Brown MP, Hauschild A, Vecchio MD, et al. An open-label, multicenter safety study of vemurafenib (PLX4032, RO5185426) in patients with metastatic melanoma. J Clin Oncol. 2012;30(Suppl). Abstract 8517.
Trefzer U, Minor D, Ribas A, Lebbe C, Siegfried A, Arya N, et al. BREAK-2: a phase IIA trial of the selective BRAF kinase inhibitor GSK2118436 in patients with BRAF (V600E/K) mutationpositive metastatic melanoma. Pigment Cell Res. 2011;24(Abstract LBA1–1):1020.
• Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–15. Molecular analysis of cutaneous SCCs and KAs in patients receiving vemurafenib indicated that mutations in RAS, particularly HRAS, are common. Provides evidence that vemurafenib may further preexisting oncogenic events.
Sinha R, Edmonds K, Newton-Bishop JA, Gore ME, Larkin J, Fearfield L. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, prevention and management of the main treatment-related skin toxicities. Br J Dermatol. 2012;167(5):987–94.
Morita H, Nagai R. Vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;365(15):1448. author reply 50.
Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30(3):316–21.
Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26(10):1657–67.
Purdie KJ, Sexton CJ, Proby CM, Glover MT, Williams AT, Stables JN, et al. Malignant transformation of cutaneous lesions in renal allograft patients: a role for human papillomavirus. Cancer Res. 1993;53(21):5328–33.
Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33–57.
Bracarda S, Ruggeri EM, Monti M, Merlano M, D’Angelo A, Ferrau F, et al. Early detection, prevention and management of cutaneous adverse events due to sorafenib: recommendations from the Sorafenib Working Group. Crit Rev Oncol Hematol. 2012;82(3):378–86.
• Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–703. Progression-free survival was significantly improved in patients receiving combined BRAF and MEK inhibitor therapy with dabrafenib and trametinib (9.4 months) compared with those receiving dabrafenib monotherapy (5.8 months). The rate of reduction of proliferative skin lesions was not significant.
Boyd KP, Vincent B, Andea A, Conry RM, Hughey LC. Nonmalignant cutaneous findings associated with vemurafenib use in patients with metastatic melanoma. J Am Acad Dermatol. 2012;67(6):1375–9.
Marquez CB, Smithberger EE, Bair SM, Wenham RM, Fenske NA, Glass LF, et al. Multiple keratoacanthomas arising in the setting of sorafenib therapy: novel chemoprophylaxis with bexarotene. Cancer Control. 2009;16(1):66–9.
Alloo A, Garibyan L, LeBoeuf N, Lin G, Werchniak A, Hodi Jr FS, et al. Photodynamic therapy for multiple eruptive keratoacanthomas associated with vemurafenib treatment for metastatic melanoma. Arch Dermatol. 2012;148(3):363–6.
• Zimmer L, Hillen U, Livingstone E, Lacouture ME, Busam K, Carvajal RD, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30(19):2375–83. First description of a systematic approach to analyzing newly developing primary cutaneous melanomas, based on mutational status and immunohistochemistry, in patients being treated with selective BRAF inhibitors. Highlights the importance of careful monitoring in these patients.
Dalle S, Poulalhon N, Debarbieux S, Thomas L. Second primary melanomas under vemurafenib. Br J Dermatol. 2012. [Epub Ahead of Print]
Ma L, Dominguez AR, Collins GR, Kia KF, Cockerell CJ. Hidradenitis Suppurativa, eruptive Melanocytic Nevi, and Keratosis Pilaris-like eruption in a patient treated with Vemurafenib. Arch Dermatol. 2012;148(12):1428–9.
Bennani-Lahlou M, Mateus C, Escudier B, Massard C, Soria JC, Spatz A, et al. Eruptive nevi associated with sorafenib treatment. Ann Dermatol Venereol. 2008;135(10):672–4.
Kong HH, Sibaud V, Chanco Turner ML, Fojo T, Hornyak TJ, Chevreau C. Sorafenib-induced eruptive melanocytic lesions. Arch Dermatol. 2008;144(6):820–2.
Balagula Y, Wu S, Su X, Feldman DR, Lacouture ME. The risk of hand foot skin reaction to pazopanib, a novel multikinase inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs. 2012;30(4):1773–81.
Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159(3):669–76.
Scandurra G, Aiello RA, Ali M, Taibi E, Sano MV, Todaro FM, et al. Appropriate management of cutaneous adverse events maximizes compliance with sorafenib treatment: a single-center experience. Future Oncol. 2012;8(5):609–15.
Maddox JS, Kung EF, Petronic-Rosic V, Sethi A. Cutaneous drug eruptions induced by sorafenib: a case series. J Drugs Dermatol. 2008;7(9):891–3.
Pomerantz RG, Mirvish ED, Geskin LJ. Cutaneous reactions to epidermal growth factor receptor inhibitors. J Drugs Dermatol. 2010;9(10):1229–34.
Balagula Y, Lacouture ME, Cotliar JA. Dermatologic toxicities of targeted anticancer therapies. J Support Oncol. 2010;8(4):149–61.
Balagula Y, Barth Huston K, Busam KJ, Lacouture ME, Chapman PB, Myskowski PL. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886). Invest New Drugs. 2011;29(5):1114–21.
Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366(9):866–8.
Gerber PA, Buhren BA, Cevikbas F, Bolke E, Steinhoff M, Homey B. Preliminary evidence for a role of mast cells in epidermal growth factor receptor inhibitor-induced pruritus. J Am Acad Dermatol. 2010;63(1):163–5.
Luu M, Boone SL, Patel J, Sullivan P, Rademaker AW, Balagula Y, et al. Higher severity grade of erlotinib-induced rash is associated with lower skin phototype. Clin Exp Dermatol. 2011;36(7):733–8.
Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemurafenib therapy. N Engl J Med. 2012;366(5):480–1.
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312–8.
Lacouture ME, Anadkat MJ, Bensadoun RJ, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19(8):1079–95.
Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol. 2008;47(2):176–86.
Lacouture ME, Reilly LM, Gerami P, Guitart J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19(11):1955–61.
Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13(9):1001–11.
Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, et al. A randomized controlled phase II study of the prophylactic effect of urea-based cream on the hand-foot skin reaction associated with sorafenib in advanced hepatocellular carcinoma. J Clin Oncol. 2012;30(Suppl). Abstract 4008.
Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist. 2009;14(3):291–302.
Infante JR, Falchook GS, Lawrence DP, Weber JS, Kefford RF, Bendell JC, et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436). J Clin Oncol. 2011;29(Suppl). CRA 8503.
Zimmer L, Livingstone E, Hillen U, Domkes S, Becker A, Schadendorf D. Panniculitis with arthralgia in patients with melanoma treated with selective BRAF inhibitors and its management. Arch Dermatol. 2012;148(3):357–61.
Degen A, Satzger I, Voelker B, Kapp A, Hauschild A, Gutzmer R. Does basal cell carcinoma belong to the spectrum of sorafenib-induced epithelial skin cancers? Dermatology. 2010;221(3):193–6.
Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61(3):522–7.
Ho PD, Zechner DK, He H, Dillmann WH, Glembotski CC, McDonough PM. The Raf-MEK-ERK cascade represents a common pathway for alteration of intracellular calcium by Ras and protein kinase C in cardiac myocytes. J Biol Chem. 1998;273(34):21730–5.
Lott JP. Vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;365(15):1449–50. author reply 50.
Conflict of Interest
Viswanath Reddy Belum declares no conflict of interest.
Alyssa Fischer declares no conflict of interest.
Jennifer Nam Choi has a speaker role with Onyx Pharmaceuticals and a consultant role with Biotest AG.
Mario E. Lacouture has a consultant role with AstraZeneca Pharmaceuticals, Roche, Bayer, Amgen, Galderma, BMS, Merck, and Pfizer. He also receives research funding from Berg, Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belum, V.R., Fischer, A., Choi, J.N. et al. Dermatological Adverse Events from BRAF Inhibitors: A Growing Problem. Curr Oncol Rep 15, 249–259 (2013). https://doi.org/10.1007/s11912-013-0308-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-013-0308-6