Abstract
Purpose of Review
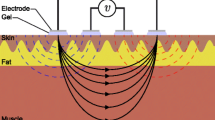
In this article, we provide an overview of electrical impedance myography (EIM), including its technical and theoretical basis, a summary of its varied applications, and ongoing developments.
Recent Findings
EIM has been used as a disease severity biomarker in a variety of disorders affecting the muscle, ranging from amyotrophic lateral sclerosis (ALS) to muscular dystrophies to disuse atrophy due to the weightlessness of space. In ALS, studies have demonstrated that major reductions in sample size in clinical trials can be achieved. Similarly, in the Duchenne muscular dystrophy, the technique tracks disease progression and is sensitive to the beneficial effect of steroids. More basic work has demonstrated that EIM can provide a non-invasive means of tracking muscle fiber size. Ongoing innovations include the development of techniques for assessing muscle contraction.
Summary
EIM is gradually being adopted as a useful, practical, and convenient tool for the assessment of neuromuscular conditions.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Grimnes S, Martinsen OG. Bioimpedance and bioelectricity basics. 3rd ed. Academic Press. 2014. p. 584
Sanchez B, Rutkove SB. Electrical impedance myography and its applications in neuromuscular disorders. Neurotherapeutics. 2017;14(1):107--118. https://doi.org/10.1007/s13311-016-0491-x.
Foster KR, Schwan HP. Dielectric properties of tissues and biological materials: a critical review. Crit Rev Biomed Eng. 1989;17:25–104.
Fricke H, Morse S. The electric resistance and capacity of blood for frequencies between 800 and 4(1/2) million cycles. J. Gen. Physiol. 1925;9:153–67.
Valdiosera R, Clausen C, Eisenberg RS. Circuit models of the passive electrical properties of frog skeletal muscle fibers. J Gen Physiol. 1974;63:432–59.
Rush S. Methods of measuring the resistivities of anisotropic conducting media in situ. J Res Natl Bur Stand Sect C Eng Instrum. 1962;66C:217.
Tsai JZ, Cao H, Tungjitkusolmun S, Woo EJ, Vorperian VR, Webster JG. Dependence of apparent resistance of four-electrode probes on insertion depth. IEEE Trans Biomed Eng. 2000;47:41–8.
Kun S, Peura R. Effects of sample geometry and electrode configuration on measured electrical resistivity of skeletal muscle. IEEE Trans Biomed Eng. 2000;47:163–9.
Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol. 1976;255:335–46.
Steendijk P, Mur G, Van Der Velde ET, Baan J. The four-electrode resistivity technique in anisotropic media: theoretical analysis and application on myocardial tissue in vivo. IEEE Trans Biomed Eng. 1993;40:1138–48.
Gielen FLH, Wallinga-de Jongeand W, Boon KL. Electrical conductivity of skeletal muscle tissue: experimental results from different muscles in vivo. Med Biol Eng Comput. 1984;22:569–77.
• Li J, Jafarpoor M, Bouxsein M, Rutkove SB. Distinguishing neuromuscular disorders based on the passive electrical material properties of muscle. Muscle Nerve. 2015;51:49–55. Electrical impedance measurements can differentiate between different categories of disease
Sanchez B, Li J, Bragos R, Rutkove SB. Differentiation of the intracellular structure of slow- versus fast-twitch muscle fibers through evaluation of the dielectric properties of tissue. Phys Med Biol. 2014;59:1–12.
Garmirian LP, Chin AB, Rutkove SB. Discriminating neurogenic from myopathic disease via measurement of muscle anisotropy. Muscle Nerve. 2009;39:16–24.
Rutkove SB, JS W, Zaidman C, Kapur K, Yim S, Pasternak A, et al. Loss of electrical anisotropy is an unrecognized feature of dystrophic muscle that may serve as a convenient index of disease status. Clin Neurophysiol. 2016;127:3546–51.
Foster K, Lukaski H. Whole-body impedance—what does it measure? Am J Clin Nutr. 1996;64:3885–965.
Tarulli AW, Garmirian LP, Fogerson PM, Rutkove SB. Localized muscle impedance abnormalities in amyotrophic lateral sclerosis. J Clin Neuromuscul Dis. 2009;10:90–6.
Rutkove SB, Shefner JM, Gregas M, Butler H, Caracciolo J, Lin C, et al. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle Nerve. 2010;42:915–21.
Spieker AJ, Narayanaswami P, Fleming L, Keel JC, Muzin SC, Rutkove SB. Electrical impedance myography in the diagnosis of radiculopathy. Muscle Nerve. 2013;48:800–5.
Rutkove SB, Geisbush TR, Mijailovic A, Shklyar I, Pasternak A, Visyak N, et al. Cross-sectional evaluation of electrical impedance myography and quantitative ultrasound for the assessment of Duchenne muscular dystrophy in a clinical trial setting. Pediatr Neurol. 2014;51:88–92. https://doi.org/10.1016/j.pediatrneurol.2014.02.015.
• Statland JM, Heatwole C, Eichinger K, Dilek N, Martens WB, Tawil R. Electrical impedance myography in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2016;54:696–701. Electrical impedance myography is sensitive to disease severity in facioscapulohumeral muscular dystrophy
Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002;25:390–7.
Rutkove SB, Zhang H, Schoenfeld DA, Raynor EM, Shefner JM, Cudkowicz ME, et al. Electrical impedance myography to assess outcome in amyotrophic lateral sclerosis clinical trials. Clin Neurophysiol. 2007;118:2413–8.
Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph Lateral Scler. 2012;13:439–45.
Shellikeri S, Yunusova Y, Green JR, Pattee GL, Berry JD, Rutkove SB, et al. Electrical impedance myography in the evaluation of the tongue musculature in amyotrophic lateral sclerosis. Muscle Nerve. 2015;52:584–91.
McIlduff CE, Yim S, Pacheck A, Geisbush T, Mijailovic ARS. An improved electrical impedance myography tongue array for use in clinical trials. Clin Neurophysiol. 2016;127:932–5.
Wang LL, Spieker AJ, Li J, Rutkove SB. Electrical impedance myography for monitoring motor neuron loss in the SOD1 G93A amyotrophic lateral sclerosis rat. Clin Neurophysiol. 2011;122:2505–11.
Li J, Sung M, Rutkove SB. Electrophysiologic biomarkers for assessing disease progression and the effect of riluzole in SOD1 G93A ALS mice. PLoS One. 2013;e65976:8.
Li J, Jafarpoor M, Bouxsein M, Rutkove SB. Distinguishing neuromuscular disorders based on the passive electrical material properties of muscle. Muscle Nerve. 2015;51(1):49–55. Published online 2014 Nov 19. https://doi.org/10.1002/mus.24270.
Ahad MA, Narayanaswami P, Kasselman LJ, Rutkove SB. The effect of subacute denervation on the electrical anisotropy of skeletal muscle: implications for clinical diagnostic testing. Clin Neurophysiol. 2010;121:882–6.
Li J, Pacheck A, Sanchez B, Rutkove SB. Single and modeled multifrequency electrical impedance myography parameters and their relationship to force production in the ALS SOD1G93A mouse. Amyotroph. Lateral Scler. Frontotemporal Degener. 2016;1–7.
Rutkove SB, Gregas MC, Darras BT. Electrical impedance myography in spinal muscular atrophy: a longitudinal study. Muscle Nerve. 2012;45:642–7.
• Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB, et al. Baseline results of the NeuroNEXT spinal muscular atrophy infant biomarker study. Ann Clin Transl Neurol. 2016;3:132–45. Electrical impedance myography is sensitive to the infantile form of spinal muscular atrophy
Arnold WD, McGovern VL, Sanchez B, Li J, Corlett KM, Kolb SJ, et al. The neuromuscular impact of symptomatic SMN restoration in a mouse model of spinal muscular atrophy. Neurobiol Dis. 2016;87:116–23.
Li J, Geisbush TR, Arnold WD, Rosen GD, Zaworski PG, Rutkove SBA. Comparison of three electrophysiological methods for the assessment of disease status in a mild spinal muscular atrophy mouse model. PLoS One. 2014;9:e111428.
Rutkove SB, Esper GJ, Lee KS, Aaron R, Shiffman CA. Electrical impedance myography in the detection of radiculopathy. Muscle Nerve. 2005;32:335–41.
Li Z, Dong T, Chen L, Wang X, Jiang L, Yu Y. Electrical impedance myography for discriminating traumatic peripheral nerve injury in the upper extremity. Clin Neurophysiol. 2017;128:384–90.
Li Z, Chen L, Zhu Y, Wei Q, Liu W, Tian D, et al. Handheld electrical impedance myography probe for assessing carpal tunnel syndrome. Ann Biomed Eng. 2017;45:1572–80.
Rutkove SB, Geisbush TR, Mijailovic A, Shklyar I, Pasternak A, Visyak N, et al. Cross-sectional evaluation of electrical impedance myography and quantitative ultrasound for the assessment of Duchenne muscular dystrophy in a clinical trial setting. Pediatr Neurol. 2014;51:88–92.
• Rutkove SB, Kapur K, Zaidman CM, Wu JS, Pasternak A, Madabusi L, et al. Electrical impedance myography for assessment of Duchenne muscular dystrophy. Ann Neurol. 2017;81:622–32. Electrical impedance myography can detect disease progression as early as 6 months in boys with muscular dystrophy as compared to healthy ones
Li J, Geisbush TR, Rosen GD, Lachey J, Mulivor A, Rutkove SB. Electrical impedance myography for the in and ex vivo assessment of muscular dystrophy (mdx) mouse muscle. Muscle Nerve. 2014;49:829–35.
Wu JS, Li J, Greenman RL, Bennett D, Geisbush T, Rutkove SB. Assessment of aged mdx mice by electrical impedance myography and magnetic resonance imaging. Muscle Nerve. 2015;52:598–604.
Sanchez B, Li J, Yim S, Pacheck A, Widrick JJ, Rutkove SB. Evaluation of electrical impedance as a biomarker of myostatin inhibition in wild type and muscular dystrophy mice. PLoS One. 2015;10(10):e0140521.
Tarulli A, Esper GJ, Lee KS, Aaron R, Shiffman CA, Rutkove SB. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology. 2005;65:451–2.
Urso ML, Clarkson PM, Price TB. Immobilization effects in young and older adults. Eur J Appl Physiol. 2006;96:564–71.
Aaron R, Esper GJ, Shiffman CA, Bradonjic K, Lee KS, Rutkove SB. Effects of age on muscle as measured by electrical impedance myography. Physiol Meas. 2006;27(10):953–9.
Kortman HG, Wilder SC, Geisbush TR, Narayanaswami P, Rutkove SB. Age- and gender-associated differences in electrical impedance values of skeletal muscle. Physiol Meas. 2013;34:1611–22.
Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–7.
Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89:81–8.
Tarulli AW, Duggal N, Esper GJ, Garmirian LP, Fogerson PM, Lin CH, et al. Electrical impedance myography in the assessment of disuse atrophy. Arch Phys Med Rehabil. 2009;90:1806–10.
Li J, Spieker AJ, Rosen GD, Rutkove SB. Electrical impedance alterations in the rat hind limb with unloading. J Musculoskelet Neuronal Interact. 2013;13:37–44.
Sung M, Li J, Spieker AJ, Spatz J, Ellman R, Ferguson VL, et al. Spaceflight and hind limb unloading induce similar changes in electrical impedance characteristics of mouse gastrocnemius muscle. J Musculoskelet Neuronal Interact. 2013;13:405–11.
Nescolarde L, Yanguas J, Lukaski H, Alomar X, Rosell-Ferrer J, Rodas G. Localized bioimpedance to assess muscle injury. Physiol Meas. 2013;34:237–45.
Nescolarde L, Yanguas J, Lukaski H, Alomar X, Rosell-Ferrer J, Rodas G. Effects of muscle injury severity on localized bioimpedance measurements. Physiol Meas. 2015;36:27–42.
Nescolarde L. Detection of muscle gap by L-BIA in muscle injuries: clinical prognosis. Physiol Measur. 2017;38(7):L1–9.
Sanchez B, Iyer SR, Li J, Kapur K, Xu S, Rutkove SB, et al. Non-invasive assessment of muscle injury in healthy and dystrophic animals with electrical impedance myography. Muscle Nerve. 2017; https://doi.org/10.1002/mus.25559.
Zaidman CM, Wang LL, Connolly AM, Florence J, Wong BL, Parsons JA, et al. Electrical impedance myography in Duchenne muscular dystrophy and healthy controls: a multicenter study of reliability and validity. Muscle Nerve. 2015;52:592–7.
NCT02611674. Methodology Study of Novel Outcome Measures to Assess Progression of ALS. Biog. Inc. 2016.
Jafarpoor M, Li J, White JK, Rutkove SB. Optimizing electrode configuration for electrical impedance measurements of muscle via the finite element method. IEEE Trans Biomed Eng. 2013;60:1446–52.
Rutkove S, Pacheck A, Sanchez B. Sensitivity distribution simulations of surface electrode configurations for electrical impedance myography. Muscle Nerve. 2017; https://doi.org/10.1002/mus.25561.
McClendon JF. The increased permeability of striated muscle to ions during contraction. Am J Physiol Leg Content. 1912;29:302–5.
Dubuisson M. Recherches sur les modifications qui survien-nent dans la conductibilité électrique du muscle au cours de la contraction. Arch Int Physiol Taylor & Francis. 1933;37:35–57.
Bozler E. The change of alternating current impedance of muscle produced by contraction. J Cell Comp Physiol. 1935;6:217–28.
Bozler E, Cole KS. Electric impedance and phase angle of muscle in rigor. J Cell Comp Physiol. 1935;6:229–41.
Sanchez B, Li J, Geisbush T, Bragos R, Rutkove S. Impedance alterations in healthy and diseased mice during electrically-induced muscle contraction. IEEE Trans Biomed Eng. 2014:1–18.
Funding
This work was supported by the National Institutes of Health R01 NS055099 and K24NS060951.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Benjamin Sanchez has received personal fees from ImpediMed, Inc., and Maxim Integrated, Inc. In addition, Dr. Sanchez has a patent 2015123603 A1 licensed to Beth Israel Deaconess Medical Center.
Seward B. Rutkove has equity in and serves a consultant and scientific advisor to Skulpt/Myolex, Inc., a company that designs impedance devices for clinical and research uses; he is also a member of the company’s board of directors. The company also has an option to license patented impedance technology of which Dr. Rutkove is named as an inventor. In addition, Dr. Rutkove has a patent US Patent #9,014,797, electrical impedance myography licensed to Skulpt, Inc.; a patent US Patent #8,892,198, devices and methods for evaluating tissue licensed to Skulpt, Inc.; a patent US patent application: a hand-held device for electrical impedance myography pending; and a patent PCT/US2015/015961, electrical impedance myography pending.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nerve and Muscle
Rights and permissions
About this article
Cite this article
Sanchez, B., Rutkove, S.B. Present Uses, Future Applications, and Technical Underpinnings of Electrical Impedance Myography. Curr Neurol Neurosci Rep 17, 86 (2017). https://doi.org/10.1007/s11910-017-0793-3
Published:
DOI: https://doi.org/10.1007/s11910-017-0793-3