Abstract
Severe sepsis and septic shock are conditions that pose difficult challenges to physicians and the health care system. In the past 10 years, a number of retrospective and prospective observational studies have shed light on the importance of a rapid and systematic approach to treatment of these conditions. A key component is early and appropriate use of antibiotics. Delay of even 6 h can dramatically increase hospital mortality. In addition, multivariate analyses have demonstrated that inappropriate initial antibiotics lead to worse outcomes. The treating physician can rapidly identify risk factors for initial inappropriate antibiotics at the bedside, such as recent antibiotic therapy or recent hospitalization. Organized antibiotic order sets have been shown to significantly improve timely appropriate antibiotic administration in septic patients. Finally, emerging laboratory data suggest that early in the course of septic shock, the pharmacokinetics of common broad spectrum antibiotics may be significantly altered due to increased volumes of distribution having dosing implications for antibiotics in septic shock.
Similar content being viewed by others
Introduction
In the first hours of a large myocardial infarction or cerebral vascular accident, time is tissue. Likewise, emerging evidence supports initiation of treatments for severe sepsis and septic shock as quickly as possible. Sepsis is a systemic inflammatory manifestation of infection associated with fever, tachycardia, elevated leukocyte count, and organ dysfunction. Severe sepsis is classified as severe end-organ damage with hypotension secondary to infectious causes. Septic shock occurs in the setting of multi-organ system failure with refractory hypotension that requires vasopressor therapy [1•, 2]. Despite advances in clinical care, mortality from severe sepsis and septic shock ranges from 20% to 50% [3–6]. The incidence of sepsis and septic shock is increasing due to an aging population with increasing risk factors, such as diabetes, chronic lung disease, long-term renal replacement therapy, human immunodeficiency virus (HIV), and immunosuppression from cancer or rheumatological therapies. Severe sepsis and septic shock remain the most common cause of death in the non-cardiac intensive care unit (ICU) [6, 7]. Cost of care in the ICU for patients with severe sepsis or septic shock is staggering and increasing [8]. Despite the broad range of insults that lead to the clinical end points of sepsis and septic shock, several consistent strategies have emerged in the last two decades that have improved ICU and hospital survival.
Clinical approaches, such as those outlined in the Surviving Sepsis Campaign, that target early therapy such as appropriate antibiotics in the disease process have been shown to reduce morbidity, mortality, and the health care costs of sepsis [1•]. International guidelines suggest infusion of broad spectrum intravenous antibiotics within the first hour that sepsis is recognized. Delay of antibiotic therapy increases the morbidity and mortality of this deadly syndrome. In addition, appropriate antibiotic therapy is equally important. The central principle of this strategy is to begin empiric antibiotics before the organism is identified by culture with a regimen based on patient risk factors for multi-resistant organisms. A strategy to initially infuse broad spectrum antibiotics involves casting a wide net on the first throw to catch the right bug. The consequences of missing the target with inappropriate antibiotics can be significant in terms of morbidity and mortality. A delay in early and appropriate antibiotic therapy in the septic patient also leads to increased health care costs [9]. This article will examine the significance of early and appropriate antibiotic therapy for severe sepsis and septic shock and provide strategies to improve outcomes in this subset of ICU patients.
Time is Tissue
In the first hours that septic shock is recognized, early aggressive management is vital. Therefore, emergency department physicians, triage nurses, and other first responders should be able to recognize appropriate signs and symptoms of sepsis and septic shock. Resuscitation with fluids, vasopressors, drawing blood/tissue cultures, and early source control with surgical debridement of suspected abscesses or removal of indwelling intravenous catheters are important to improve survival [1•, 10]. However, early therapy with broad spectrum antibiotics is a central theme of critical care management for these patients. This strategy of rapid infusion of antibiotics was initially recognized in the management of bacterial meningitis as well as among febrile neutropenic patients with hematological malignancies [11–14]. In a retrospective study by Kumar et al. [15] among patients with septic shock in 2006, each hour antibiotics are delayed when septic shock has been recognized increased mortality by 7.6%. Overall survival if antibiotics were delayed by 6 h was 42% compared to 79.9% if antibiotics were given within the first hour. Multivariate analysis revealed that among factors such as early fluid resuscitation, number of antibiotics, or the presence of vasopressor therapy, only the delay from hypotension to first dose of antibiotics was strongly associated with mortality regardless of infection site or type of organism. Subsequently, Kumar et al. [16••] demonstrated this principle in all infectious sources, all epidemiological subgroups, and across all microorganisms. A larger prospective multicenter observational study of 2804 patients from 77 ICUs in Europe determined that after implementation of the Surviving Sepsis Guidelines, early antibiotic therapy within the first 6 h of presentation was associated with significant improvement in hospital mortality [17•].
The Right Drug for the Right Bug
Inappropriate antibiotic usage occurs when health care workers fail to recognize risk factors for hospital-associated infections such as Pseudomonas aeruginosa, other resistant Gram-negative organisms, methicillin-resistant Staphylococcus aureus (MRSA), or fungal infections. Given the importance of early antibiotic therapy, this recognition needs to occur among first responders in the emergency department or on the hospital ward. Appropriate antibiotic use involves the timely infusion of the correct drug most likely to treat the suspected organism with sufficient in vitro sensitivity while avoiding drug toxicities and interactions [18, 19]. Over 2 decades of research has identified the significance of the appropriateness of the initial antibiotics infused in the first hours of septic shock. In a 1999 prospective single-center cohort study of adult patients with blood stream infections in the ICUs of our institution, hospital mortality was significantly lower (12.2%) among those who received appropriate antibiotics compared to those who received inappropriate antibiotics (52.1%) (relative risk [RR], 4.26; 95% confidence interval [CI], 3.52–5.15; P < 0.001) [19]. Moreover, multivariate analysis of this cohort demonstrated that initial inappropriate antibiotics was the most important risk factor for hospital mortality (adjusted odds ratio 4.26; 95% CI 3.35–5.44; P < 0.001). A follow-up analysis of a larger cohort from the same institution confirmed these findings [20]. In addition, this study illustrated the significance of hospital-acquired versus community-acquired infections. Inappropriate antibiotics were more common among patients with hospital-acquired infections (35%) and particularly common in those who were admitted for a community-acquired infection and later developed a secondary hospital-acquired infection (75%) compared to community-acquired infection alone (20%) (P < 0.001).
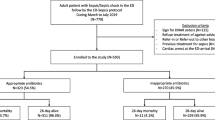
The significance of early appropriate antibiotics was confirmed in a retrospective single center study among 406 adult ICU non-surgical patients with sepsis, severe sepsis, or septic shock [21]. Multivariate logistic regression analysis confirmed that patients who received inappropriate antibiotics had lower hospital survival (28.2%) compared to those who received appropriate antibiotic therapy (46.7%). The authors also noted that patients with sepsis or severe sepsis that were treated with inappropriate antibiotics were more likely to develop septic shock. The initial infusion of appropriate antibiotics in patients presenting with severe sepsis or septic shock has now been shown to be a significant determinant of hospital survival across different patient cohorts over the last 10 years (Fig. 1).
At the onset of a serious bacterial infection that results in sepsis, the treatment strategy is based on the suspected pathogens and antibiotic resistance patterns. Community-acquired infections, and hospital-acquired infections are typically caused by different pathogens. Healthcare-associated infections involve patients admitted to the hospital from the community that have one or more risk factors such as the need for hemodialysis, home health care services, nursing home care, recent hospitalization within 90 days, recent antibiotic usage, or chronic immunosuppression. This group of patients, while coming from the community, are frequently infected with organisms found in hospital-acquired infections such as MRSA, fungi, Pseudomonas aeruginosa, or other resistant Gram-negative bacteria [18, 22, 23]. The percentage of patients with healthcare-associated risk factors is a rapidly growing segment of the healthcare population. Finally, patients with hospital-acquired and healthcare-associated infections are also more likely to be treated with inappropriate antibiotics and are at higher risk for developing infections with bacteria resistant to standard commonly employed antibiotics as demonstrated at our institution for cefepime, meropenem or piperacillin/ tazobactam [24••].
A number of risk factors have been identified that are related with initial infusion of inappropriate antibiotics (Table 1). As mentioned above, the presence of invasive fungal infections, resistant organisms such as enterococcal species, MRSA, and extended-spectrum β-lactamase (ESBL) producing organisms are associated with higher risk of inappropriate antibiotic therapy and higher mortality. Getting the antibiotic right in the first few hours of illness is vital. Altering antibiotic therapy when the subsequent culture data become available frequently is too little…too late. Yet prediction of these resistant or atypical organisms at the onset of illness is often difficult. Rather, the treating physician can quickly assess two practical factors from a glance that are associated with initial inappropriate antibiotic infusion: the presence of prior antibiotic treatment with any antibiotic within 30–60 days of presentation and development of the infection in the hospital or in patients with healthcare-associated risk factors [20, 21].
If One Appropriate Antibiotic is Good, are Two Better?
Gram negative infections, particularly those caused by Pseudomonas aeruginosa or by ESBL producing organisms, are significant causes of healthcare-associated and hospital-acquired infections. Infections with these multi-drug resistant Gram negative organisms are a frequent cause of inappropriate initial antibiotics. Previous studies have suggested that initial combination antibiotic therapy may be more effective and reduce the emergence of resistance in high risk cases of ventilator-associated pneumonia [23, 25, 26] and among patients with neutropenic sepsis [27, 28]. A recent retrospective study of 760 patients from our institution with severe sepsis or septic shock attributed to Gram negative infection indicated that those patients with healthcare-associated or hospital-acquired infections, were more likely to be infected with resistant organisms, and had a greater hospital mortality rate due to treatment with inappropriate initial antibiotics [24••]. Outcomes were compared between those treated with initial monotherapy versus initial combination antibiotics. In that study, typical empiric monotherapy for Gram negative infections included either cefepime, an anti-pseudomonal carbapenem, or piperacillin-tazobactam. Combination therapy included any of the afore mentioned antibiotics combined with either a fluoroquinolone (ciprofloxacin) or an aminoglycoside (gentamicin). Combination therapy improved the appropriateness of therapy by increasing susceptibility of the antibiotic regimen. Empiric combination antibiotic therapy directed against Gram negative infections led to a reduction in inappropriate antibiotic administration (22.2% vs. 36%, P < 0.001). The effect was most pronounced in those receiving combination therapy with gentamicin.
A large multicenter retrospective analysis of a cohort of 4662 patients with septic shock compared outcomes with initial monotherapy versus initial combination antibiotic therapy (β-lactam plus a fluoroquinolone, an aminoglycoside, a macrolide, or clindamycin) [29••]. In this study, a β-lactam plus a glycopeptides such as vancomycin was not considered to be combination therapy. In addition to increasing the appropriateness of the antibiotic regimen, this strategy led to a survival benefit. Initial combination therapy was associated with reduced 28 day mortality (36.3% vs 29.0%; hazard ratio, 0.77; 95% CI, 0.67–0.88; P = 0.0002). The more rapid the second antibiotic was infused after development of hypotension the greater the absolute risk reduction in mortality (10% vs 2.5%). Furthermore, vasopressor dependence and requirement for mechanical ventilation were also decreased in the combination antibiotic group.
Despite these two studies, the initial use of empiric combination antibiotics remains controversial. The greater number of antibiotics infused may increase the risk of toxic side effects, such as renal toxicity from an aminoglycoside, or the development of antibiotic-associated colitis. Furthermore, a large meta-analysis failed to demonstrate a significant improvement in outcomes for sepsis with combination antibiotic therapy [30•].This meta-analysis included populations with greater heterogeneity of clinical severity (mean fatality rate was 8.6%) compared to the above two studies that were restricted to severe sepsis and septic shock [24••, 29••]. A second caveat among these different studies is the purpose for which combination antibiotics are given; either to ensure appropriateness of initial therapy or to provide a prolonged synergistic effect as is utilized in the treatment of endocarditis. It is our practice to utilize initial combination antibiotics for patient with sepsis and septic shock when the appropriateness of a single agent is in doubt. Commonly, this is employed by the addition of one or perhaps two doses of an aminoglycoside up front with either a 4th generation cephalosporin or a carbapenem. When the culture and susceptibility data have returned, the antibiotics are then adjusted accordingly. Long-term synergistic dosing of two or more antibiotics is not necessarily the goal. Knowledge of the local susceptibility patterns of common Gram negative infections can be of assistance. For example, patients from a particular ICU at a large hospital known to have Gram negative infections that historically have high resistance to cephalosporins may benefit from the initial addition of an aminoglycoside until the in vitro susceptibility data are available.
Bundled Therapy
In a busy emergency department or ICU, it may difficult to ensure timely appropriate antibiotic coverage for patients with early septic shock. Bundled hospital orders have been successfully implemented as a means to improve the rate of therapy with appropriate initial antibiotics. This approach was initially successful for standardizing treatment for acute myocardial infarction [31, 32]. The early directed goal therapy approach for resuscitation in the emergency department of patients with septic shock demonstrated a decrease in mortality with physician order sets that directed management of hypotension from sepsis [33]. Likewise, use of the Surviving Sepsis Campaign guidelines for the treatment of septic shock, particularly early appropriate antibiotics, reduced hospital mortality in a large prospective study [17].
At our hospital, a bundled antibiotic order set was devised and tested in the emergency department. A cohort of 120 patients presenting with symptoms of septic shock (mean APACHE II score 22.5 +/− 8.3) were prospectively studied before and after implantation of the antibiotic order sets. The patients in the after-cohort were more likely to receive appropriate antibiotics (86.7% versus 71.7%). The probability of 28 day hospital survival was also significantly increased (P < 0.001 by log rank test) [34]. Further analysis of this cohort revealed that patients who received early initial antibiotics via the order set in the emergency department had shorter duration of hospitalization and significant reductions in hospitalization costs (P = 0.009) [9]. An analysis of over 15,000 patient from 252 sites around the world confirmed that use of order sets based on the Surviving Sepsis Campaign guidelines, including early, appropriate antibiotics, were associated with a significant reduction in hospital mortality (odds ratio, 0.86; 95% CI, 0.79–0.93; P < 0.0001) [35••]. This approach was also confirmed in a recent meta-analysis of bundled care for patients with septic shock demonstrating reduced hospital mortality in all studies examined [36•]. Three measures of antibiotic use, time to initial infusion, appropriate antibiotics, and timely infusion of antibiotics were all significantly improved with bundled therapy and associated with improved outcomes.
Appropriate Initial Antibiotic Dosing
In addition to the early infusion of antibiotics in septic shock, the proper dosage is also critical. From a pharmacokinetic perspective, antibiotic chemotherapy is divided into loading and maintenance dosing. The physiology of early sepsis alters the typical pharmacokinetics of the loading dose due to increased volume of distribution (Vd) in the extracellular space from intravenous fluid resuscitation, renal or hepatic failure, or increased cardiac output [37, 38•]. The biochemical properties of the antibiotic also determine the plasma and local tissue concentrations. The source of infection determines the dose required to achieve adequate minimal inhibitory concentrations (MICs) of the antibiotic at the tissue level (lung, blood, or intra-abdominal). Therefore in severe life threatening infections, maximum first dose antibiotics are important. Multiple studies have described that hydrophilic antibiotics such as β-lactams, aminoglycosides, piperacillin-tazobactam, or vancomycin require higher loading doses to achieve sufficient levels at the site of the infection in the interstitial space [39–41]. This requirement was irrespective of underlying hepatic or renal failure. The dilution effect in the extracellular space is less significant in the dosing of lipophilic antibiotics such as fluoroquinolones and linezolid [37, 42].
In vivo models suggest that β-lactams have a time depended anti-microbial mechanism with an optimal bactericidal effect when the drug concentration is greater than four times the MIC of the pathogen [43, 44]. One study compared the first dose pharmacokinetics of four typical β-lactam antibiotics in 80 patients admitted to 4 Belgium ICUs for bactremia, 72% with septic shock [45••]. Among, meropenem, ceftazidime, cefepime, and piperacillin-tazobactam, cefepime and piperacillin-tazobactam had the shortest periods with a serum concentration greater than four times the MIC after the first dose. In contrast meropenem had the longest time with a concentration greater than four times the MIC. Not surprisingly, pathogens with higher MICs had the shortest period with concentrations greater than 4 times the MIC. There has been speculation that for antibiotics such as piperacillin-tazobactam without a significant post-antibiotic effect, more frequent infusions or even continuous infusions might be of benefit. However a recent meta-analysis of continuous versus bolus infusion of β-lactam antibiotics for treatment of infections failed to identify a mortality benefit (OR, 1.00; 95% CI, 0.48-2.06; P = 1.00) [46]. While continuous or prolonged infusion of antibiotic therapy may be preferred from a pharmacokinetic standpoint, practical challenges often supersede. Early in the management of septic shock, vascular access may be limited, and intravenous fluids and vasopressors are often continuously required. Future studies are needed to allow for real-time adjustments for antibiotic dosing in the septic patient that does not rely on pending culture results or MIC data in order to optimize the administration of therapy.
Conclusions
In the first moments of severe sepsis and septic shock, several factors guide the practice of antibiotic therapy. The time of onset of hypotension sets the clock running in septic shock.
-
Timely infusion of antibiotics within 2 h or less of onset of hypotension seems critical.
-
Review for risk factors for inappropriate antibiotic administration is necessary (eg, prior antibiotic administration, healthcare-associated risk factors).
-
Avoidance of treatment with the same antibiotics for new infection less than 30–60 days following treatment for a prior bacterial infection.
-
Consider initial combination antibiotics to ensure culture susceptibility not necessary for synergy.
-
Utilize bundled antibiotic order sets to improve compliance with treatment guidelines.
Despite improvements in care of critically ill patients with septic shock, the overall incidence, disease severity, and mortality of this patient population has increased [6]. Critical care medicine has to rise to meet this challenge. Initiation of rapid response measures centered on early and appropriate use of antibiotic therapy is the cornerstone of this strategy. Critical care and emergency department physicians have one chance to get the right drug for the likely pathogen infused as rapidly as possible. Bundled sets of antibiotic orders facilitate this process by increasing the appropriateness of initial antibiotic therapy. Further research to improve initial antibiotic dosing by bedside personalized pharmacokinetics may help to optimize the effectiveness of this therapy for each patient.
References
Recently published papers of particular importance are highlighted as follows: • Of importance •• Of major importance
• Dellinger RP, Zimmerman JL, Vincent JL, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296–327. A thorough summary of important guidelines in the diagnosis and management of septic shock.
Vincent JL, Korkut HA. Defining sepsis. Clin in Chest Med. 2008;29:585–90.
Alberti C, Brun-Buisson H, Le Gall R, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–21.
Vincent JL, Sakr Y, Payen D, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53.
Martin GS, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. NEJM. 2003;348:1546–54.
Drombrovskiy VY, Sunderram J, Martin AA, Paz HL. Rapid Increase in hospitalization and mortality rates for severe sepsis in the United States. Crit Care Med. 2007;35:1244–50.
Angus D, Wax R. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–16.
Burchardi H, Schneider H. Economic aspects of severe sepsis: a review of intensive care unit cost of illness and cost effectiveness of therapy. Pharmacoeconomics. 2004;22:793–813.
Shorr AF, Micek S, Jackson WL, Kollef MH. Economic implications of an evidence-based sepsis protocol: can we improve outcomes and lower costs? Crit Care Med. 2007;35:1257–62.
Oterro RM, Nuygen HB, Rivers E, et al. Early goal-directed therapy in severe sepsis and septic shock revisited: concepts, controversies, and contemporary findings. Chest. 2006;130:1579–95.
Talan DA, Zibulewky J. Relationship of clinical presentation to time to antibiotics for the emergency department management of suspected bacterial meningitis. Ann Emerg Med. 1993;22:1733–8.
Lebel MH. Adverse outcome of bacterial meningitis due to delayed sterilization of cerebrospinal fluid. Antibiot Chemother. 1992;45:226–38.
Miner J, Heegaard W, Mapes A, Biros M. Presentation, time to antibiotics, and mortality of patients with bacterial meningitis at an urban county medical center. J Emerg Med. 2001;21:387–92.
Larché J, Azoulay E, Schlemmer B, et al. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29:1688–95.
Kumar A, Roberts D, Cheang M, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96.
•• Kumar A, Ellis P, Chateau D, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009;136:1237–48. Authors demonstrated that timely infusion and appropriate antibiotics had a profound effect on hospital mortality from severe sepsis and septic shock independent of other co morbid conditions.
• Ferrer R, Artigas A, Sirvent JM, et al. Effectiveness of treatments for severe sepsis. Am J Resp Crit Care Med 2009;180:861–66. In a ICU patient cohort after the implementation of the Surviving Sepsis Guidelines, administration of timely appropriate antibiotics and drotrecogin-α were associated with reduced mortality from septic shock.
Kollef MH, Morrow LE, Baughman RP, et al. Health care-associated pneumonia (HCAP): a critical appraisal to improve identification, management, and outcomes–proceedings of the HCAP Summit. Clin Infect Dis. 2008;46:S296–334.
Kollef MH, Sherman G, Ward S, Fraser V. Inadequate antimicrobial treatment of infections. Chest. 1999;115:462–74.
Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55.
Garnacho-Montero J, Garcia-Garmendia JL, Oritz-Leyba C, et al. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31:2742–51.
Friedman ND, Kayes KS, Sexton DJ, et al. Healthcare-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7.
Niederman MS, Craven DE. ATS Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Resp Crit Care Med. 2005;171:388–416.
•• Micek S, Welch E, Kollef MH, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: A retrospective analysis. Antimicrob Agents Chemother 2010;54:1742–48. A single center study of serious Gram negative blood stream infections demonstrated initial combination antibiotics decreased the risk of receiving inappropriate antibiotics. A higher mortality and increased hospital length of stay for those receiving inappropriate antibiotics.
Heyland D, Dodek P, Cook K, et al. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Car Med. 2008;36:737–44.
Beardsley J, Williamson JC, Bowton DL, et al. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest. 2006;130:787–93.
Dejace P, Klastersky J. Comparative review of combination therapy: two beta-lactams versus beta-lactam plus aminoglycoside. Am J Med. 1986;80:29–38.
Bodey GP. Evolution of antibiotic therapy for infection in neutropenic patients: studies at M.D. Anderson Hospital. Review Infect Dis. 1989;11:S1582–90.
•• Kumar A, Zarychanski R, Doucette S, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Chest 2010;38:1773–85. This study demonstrated that initial combination antibiotics for septic shock increased 28-day survival (P = 0.0002) along with pressor free and ventilator free days in the ICU.
• Mical P, Simona G, Leibovici L, et al. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Sys Rev 2006;25:CD003344. A large meta-analysis of over 7000 patients with sepsis, pneumonia, endocarditis, and other clinical conditions revealed no benefit to initial combination antibiotics compared to monotherapy.
Hamm C, Goldmann B, Meinertz T, et al. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. NEJM. 1997;337(23):1648–53.
Ryan TJ, Antman EM, Brooks NH, et al. 1999 update: ACC/AHA Guidelines for the management of patients with acute myocardial infarction: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). Circulation. 1999;100:1016–30.
Rivers E, Nguyen B, Peterson E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. NEJM. 2001;345:1368–77.
Micek S, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Car Med. 2006;34:2707–13.
•• Levy MM, Dellinger RP, Regen S, et al. The surviving Sepsis Campaign: results of an international guideline based performance improvement program targeting severe sepsis. Intensive Care Med 2010;36:222–31. This large study of patients with severe sepsis compared outcomes after the initiating The Surviving Sepsis Guidelines.
• Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med 2010;38:668–78. The authors compared multiple studies of the impact of bundled order sets for septic shock. Post-intervention groups had consistent increase in survival and timely, appropriate antibiotic use.
Sharma S, Kumar A. Antimicrobial management of sepsis and septic shock. Clin Chest Med. 2008;29:677–87.
• Pea F, Viele P. Bench-to-bedside review: appropriate antibiotic therapy in severe sepsis and septic shock—does the dose matter? Crit Care 2009;13:214. An excellent review of the pharmacokinetic and pharmacodynamic changes of antibiotic therapy for severe sepsis and septic shock.
Rea RS, Capitano B, Smith R, et al. Suboptimal aminoglycoside dosing in critically ill patients. Ther Drug Monit. 2008;30:674–81.
Joukhadar C, Frossard M, Mayer BX, et al. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit Care Med. 2001;29:385–91.
del Mar Fernandez de Gatta Garcia M, Revilla N, Calvo MV, et al. Pharmacokinetic/ pharmacodynamic analysis of vancomycin in ICU patients. Intensive Care Med 2007;33:279–85.
Varghese JM, Roberts JA, Lipman J. Antimicrobial pharmacokinetic and pharmacodynamic issues in the critically ill with severe sepsis and septic shock. Crit Care Clin. 2011;27:19–34.
Tam VH, McKinnon PS, Akins RL, et al. Pharmacodynamics of cefepime in patients with Gram-negative infections. J AntimicrobChemother. 2002;50:425–8.
McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T > MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31:345–51.
•• Taccone FS, Laterre PF, Vincent JL, et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care Med 2010;14:R126. A prospective study of the pharmacokinetics of 4 common b-lactams administered as first dose antibiotics for septic shock revealed only meropenem reached the target serum MIC for pseudomonas infections.
Roberts JA, Webb S, Paterson D, et al. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37:2071–8.
Harbarth S, Garbino J, Lew D, et al. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529–35.
Zaragoza R, Artero A, Camarena J, et al. The influence of inadequate empirical antimicrobial treatment on patient with blood stream infections in an intensive care unit. Clin Microbiol Infect. 2003;9:412–8.
Leone M, Bourgoin A, Martin C, et al. Empirical antimicrobial therapy of septic shock patients: adequacy and impact on the outcome. Crit Care Med. 2003;31:462–7.
Shorr AF, Micek S, Reichley RM, Kollef MH. Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit Care Med. 2011;39:46–51.
Acknowledgement
Dr. Kollef’s efforts were supported by the Barnes-Jewish Hospital Foundation
Disclosure
Marin H. Kollef has received payment for consultancy and development of educational presentations from Cubist, Merck, Pfizer, and Hospira; John D. Dickinson reported no potential conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dickinson, J.D., Kollef, M.H. Early and Adequate Antibiotic Therapy in the Treatment of Severe Sepsis and Septic Shock. Curr Infect Dis Rep 13, 399–405 (2011). https://doi.org/10.1007/s11908-011-0206-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-011-0206-8