Abstract
Purpose of Review
The study aims to review recent advances in knowledge on the interplay between miRNAs and the sex-determining Region Y (SRY)-related high-mobility-group box 6 (Sox6) in physiology and pathophysiology, highlighting an important role in autoimmune and cardiometabolic conditions.
Recent Findings
The transcription factor Sox6 is an important member of the SoxD family and plays an indispensable role in adult tissue homeostasis, regeneration, and physiology. Abnormal expression of the Sox6 gene has been implicated in several disease conditions including diabetes, cardiomyopathy, autoimmune diseases, and hypertension. Expression of Sox6 is regulated by miRNAs, which are RNAs of about 22 nucleotides, and have also been implicated in several pathophysiological conditions where Sox6 plays a role.
Summary
Regulation of Sox6 by miRNAs is important in diverse physiological tissues and organs. Dysregulation of the interplay between miRNAs and Sox6 is an important determinant of various disease conditions and may be actionable for therapeutic purposes.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ma Z, Jin X, He L, Wang Y. CXCL16 regulates renal injury and fibrosis in experimental renal artery stenosis. Am J Physiol Heart Circ Physiol. 2016;311(3):H815–21. https://doi.org/10.1152/ajpheart.00948.2015.
Roose J, Korver W, Oving E, Wilson A, Wagenaar G, Markman M, et al. High expression of the HMG box factor sox-13 in arterial walls during embryonic development. Nucleic Acids Res. 1998;26(2):469–76. https://doi.org/10.1093/nar/26.2.469.
Takamatsu N, Kanda H, Tsuchiya I, Yamada S, Ito M, Kabeno S, et al. A gene that is related to SRY and is expressed in the testes encodes a leucine zipper-containing protein. Mol Cell Biol. 1995;15(7):3759–66. https://doi.org/10.1128/mcb.15.7.3759.
Connor F, Wright E, Denny P, Koopman P, Ashworth A. The Sry-related HMG box-containing gene Sox6 is expressed in the adult testis and developing nervous system of the mouse. Nucleic Acids Res. 1995;23(17):3365–72. https://doi.org/10.1093/nar/23.17.3365.
•• Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227(2):239–55. https://doi.org/10.1006/dbio.2000.9883. This study reviewed the evolutionary history of the members of the Sox family using complete HMG domain sequencing data and protein structure.
Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3(2):167–70. https://doi.org/10.1016/s1534-5807(02)00223-x.
Phochanukul N, Russell S. No backbone but lots of Sox: invertebrate Sox genes. Int J Biochem Cell Biol. 2010;42(3):453–64. https://doi.org/10.1016/j.biocel.2009.06.013.
Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 2000;16(4):182–7. https://doi.org/10.1016/s0168-9525(99)01955-1.
Lefebvre V. The SoxD transcription factors—Sox5, Sox6, and Sox13—are key cell fate modulators. Int J Biochem Cell Biol. 2010;42(3):429–32. https://doi.org/10.1016/j.biocel.2009.07.016.
Hiraoka Y, Ogawa M, Sakai Y, Kido S, Aiso S. The mouse Sox5 gene encodes a protein containing the leucine zipper and the Q box. Biochim Biophys Acta. 1998;1399(1):40–6. https://doi.org/10.1016/s0167-4781(98)00086-4.
Han Y, Lefebvre V. L-Sox5 and Sox6 drive expression of the aggrecan gene in cartilage by securing binding of Sox9 to a far-upstream enhancer. Mol Cell Biol. 2008;28(16):4999–5013. https://doi.org/10.1128/MCB.00695-08.
Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17(19):5718–33. https://doi.org/10.1093/emboj/17.19.5718.
Stolt CC, Schlierf A, Lommes P, Hillgartner S, Werner T, Kosian T, et al. SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev Cell. 2006;11(5):697–709. https://doi.org/10.1016/j.devcel.2006.08.011.
Stolt CC, Lommes P, Hillgartner S, Wegner M. The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Res. 2008;36(17):5427–40. https://doi.org/10.1093/nar/gkn527.
Hou L, Srivastava Y, Jauch R. Molecular basis for the genome engagement by Sox proteins. Semin Cell Dev Biol. 2017;63:2–12. https://doi.org/10.1016/j.semcdb.2016.08.005.
Leow SC, Poschmann J, Too PG, Yin J, Joseph R, McFarlane C, et al. The transcription factor SOX6 contributes to the developmental origins of obesity by promoting adipogenesis. Development. 2016;143(6):950–61. https://doi.org/10.1242/dev.131573.
Li X, Wang J, Jia Z, Cui Q, Zhang C, Wang W, et al. MiR-499 regulates cell proliferation and apoptosis during late-stage cardiac differentiation via Sox6 and cyclin D1. PLoS One. 2013;8(9): e74504. https://doi.org/10.1371/journal.pone.0074504.
Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65(5):612–26. https://doi.org/10.1016/j.neuron.2010.02.018.
Yi Z, Cohen-Barak O, Hagiwara N, Kingsley PD, Fuchs DA, Erickson DT, et al. Sox6 directly silences epsilon globin expression in definitive erythropoiesis. PLoS Genet. 2006;2(2): e14. https://doi.org/10.1371/journal.pgen.0020014.
Iguchi H, Ikeda Y, Okamura M, Tanaka T, Urashima Y, Ohguchi H, et al. SOX6 attenuates glucose-stimulated insulin secretion by repressing PDX1 transcriptional activity and is down-regulated in hyperinsulinemic obese mice. J Biol Chem. 2005;280(45):37669–80. https://doi.org/10.1074/jbc.M505392200.
Saleem M, Barturen-Larrea P, Gomez JA. Emerging roles of Sox6 in the renal and cardiovascular system. Physiol Rep. 2020;8(22): e14604. https://doi.org/10.14814/phy2.14604.
•• Hagiwara N. Sox6, jack of all trades: a versatile regulatory protein in vertebrate development. Dev Dyn. 2011;240(6):1311–21. https://doi.org/10.1002/dvdy.22639. This review described the versatile nature of Sox6 in details during early developmental stages in vertebrates.
Jiang ZH, Tang YZ, Song HN, Yang M, Li B, Ni CL. miRNA342 suppresses renal interstitial fibrosis in diabetic nephropathy by targeting SOX6. Int J Mol Med. 2020;45(1):45–52. https://doi.org/10.3892/ijmm.2019.4388.
Qi H, Yao L, Liu Q. MicroRNA-96 regulates pancreatic beta cell function under the pathological condition of diabetes mellitus through targeting Foxo1 and Sox6. Biochem Biophys Res Commun. 2019;519(2):294–301. https://doi.org/10.1016/j.bbrc.2019.09.001.
•• Ding J, Chen J, Wang Y, Kataoka M, Ma L, Zhou P, et al. Trbp regulates heart function through microRNA-mediated Sox6 repression. Nat Genet. 2015;47(7):776–83. https://doi.org/10.1038/ng.3324. This study showed that Sox6 has a role in cardiomyopathy. Using cardiomyocyte specific Trbp (Tarbp2) knockout mice, it showed that inactivation of Trbp results in increasing expression of Sox6 causing progressive cardiomyopathy and heart failure. Overexpression of Mir208a repressed Sox6 expression and rescued cardiac function in Trbp KO mice.
Hagiwara N, Klewer SE, Samson RA, Erickson DT, Lyon MF, Brilliant MH. Sox6 is a candidate gene for p100H myopathy, heart block, and sudden neonatal death. Proc Natl Acad Sci U S A. 2000;97(8):4180–5. https://doi.org/10.1073/pnas.97.8.4180.
Wang J, Ding S, Duan Z, Xie Q, Zhang T, Zhang X, et al. Role of p14ARF-HDM2-p53 axis in SOX6-mediated tumor suppression. Oncogene. 2016;35(13):1692–702. https://doi.org/10.1038/onc.2015.234.
Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175(2):372–86 e17. https://doi.org/10.1016/j.cell.2018.08.067.
•• McCarthy JJ, Esser KA, Peterson CA, Dupont-Versteegden EE. Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy. Physiol Genomics. 2009;39(3):219–26. https://doi.org/10.1152/physiolgenomics.00042.2009. An early study showing that gene expression in skeletal muscle is dependent on an interplay between miRNAs and Sox6.
Sluijter JP, van Mil A, van Vliet P, Metz CH, Liu J, Doevendans PA, et al. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30(4):859–68. https://doi.org/10.1161/ATVBAHA.109.197434.
Duran B, Dal-Pai-Silva M, Garcia de la Serrana D. Rainbow trout slow myoblast cell culture as a model to study slow skeletal muscle, and the characterization of mir-133 and mir-499 families as a case study. J Exp Biol. 2020;223(Pt 2). https://doi.org/10.1242/jeb.216390.
Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–98. https://doi.org/10.1016/s0092-8674(03)01018-3.
Goljanek-Whysall K, Sweetman D, Munsterberg AE. microRNAs in skeletal muscle differentiation and disease. Clin Sci (Lond). 2012;123(11):611–25. https://doi.org/10.1042/CS20110634.
van Rooij E, Liu N, Olson EN. MicroRNAs flex their muscles. Trends Genet. 2008;24(4):159–66. https://doi.org/10.1016/j.tig.2008.01.007.
Fagard M, Boutet S, Morel JB, Bellini C, Vaucheret H. AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc Natl Acad Sci U S A. 2000;97(21):11650–4. https://doi.org/10.1073/pnas.200217597.
Khan J, Lieberman JA, Lockwood CM. Variability in, variability out: best practice recommendations to standardize pre-analytical variables in the detection of circulating and tissue microRNAs. Clin Chem Lab Med. 2017;55(5):608–21. https://doi.org/10.1515/cclm-2016-0471.
•• Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54. https://doi.org/10.1016/0092-8674(93)90529-y. Early description of the existence of non-coding RNAs.
Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10(12):1957–66. https://doi.org/10.1261/rna.7135204.
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23(20):4051–60. https://doi.org/10.1038/sj.emboj.7600385.
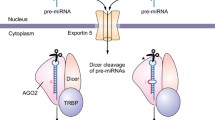
Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21(17):4663–70. https://doi.org/10.1093/emboj/cdf476.
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–9. https://doi.org/10.1038/nature01957.
Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–6. https://doi.org/10.1101/gad.1158803.
Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106(1):23–34. https://doi.org/10.1016/s0092-8674(01)00431-7.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. https://doi.org/10.1016/s0092-8674(04)00045-5.
Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, et al. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65(5):597–611. https://doi.org/10.1016/j.neuron.2010.01.027.
Wu L, Belasco JG. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell. 2008;29(1):1–7. https://doi.org/10.1016/j.molcel.2007.12.010.
Bartel DP. Metazoan MicroRNAs. Cell. 2018;173(1):20–51. https://doi.org/10.1016/j.cell.2018.03.006.
Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015;16(7):421–33. https://doi.org/10.1038/nrg3965.
• Ipsaro JJ, Joshua-Tor L. From guide to target: molecular insights into eukaryotic RNA-interference machinery. Nat Struct Mol Biol. 2015;22(1):20–8. https://doi.org/10.1038/nsmb.2931. This reviewed studies that showed the versatility of RNA-induced silencing complexes and emphasized the importance of both upstream biogenesis and downstream silencing factors. In addition, it focuses on describing and depicting the mechanisms and structures that govern RNA silencing in higher organisms.
Lee R, Feinbaum R, Ambros V. A short history of a short RNA. Cell. 2004;116(2 Suppl):S89–92, 1 following S6. https://doi.org/10.1016/s0092-8674(04)00035-2.
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–743. https://doi.org/10.1161/CIR.0000000000000950.
Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest. 2009;119(9):2772–86. https://doi.org/10.1172/JCI36154.
Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, et al. Altered microRNA expression in human heart disease. Physiol Genomics. 2007;31(3):367–73. https://doi.org/10.1152/physiolgenomics.00144.2007.
van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316(5824):575–9. https://doi.org/10.1126/science.1139089.
Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436(7048):214–20. https://doi.org/10.1038/nature03817.
•• Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol. 2016;68(23):2577–84. https://doi.org/10.1016/j.jacc.2016.09.945. Description of the network of MyomiR, a subgroup of miRNAs regulating differentiation of skeletal and cardiac muscle.
Yeung F, Chung E, Guess MG, Bell ML, Leinwand LA. Myh7b/miR-499 gene expression is transcriptionally regulated by MRFs and Eos. Nucleic Acids Res. 2012;40(15):7303–18. https://doi.org/10.1093/nar/gks466.
Shieh JT, Huang Y, Gilmore J, Srivastava D. Elevated miR-499 levels blunt the cardiac stress response. PLoS One. 2011;6(5): e19481. https://doi.org/10.1371/journal.pone.0019481.
Quiat D, Voelker KA, Pei J, Grishin NV, Grange RW, Bassel-Duby R, et al. Concerted regulation of myofiber-specific gene expression and muscle performance by the transcriptional repressor Sox6. Proc Natl Acad Sci U S A. 2011;108(25):10196–201. https://doi.org/10.1073/pnas.1107413108.
van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17(5):662–73. https://doi.org/10.1016/j.devcel.2009.10.013.
Yousefzadeh N, Jeddi S, Ghiasi R, Alipour MR. Effect of fetal hypothyroidism on MyomiR network and its target gene expression profiles in heart of offspring rats. Mol Cell Biochem. 2017;436(1–2):179–87. https://doi.org/10.1007/s11010-017-3089-7.
Huang X, Li Z, Bai B, Li X, Li Z. High expression of microRNA-208 is associated with cardiac hypertrophy via the negative regulation of the sex-determining region Y-box 6 protein. Exp Ther Med. 2015;10(3):921–6. https://doi.org/10.3892/etm.2015.2645.
Azibani F, Devaux Y, Coutance G, Schlossarek S, Polidano E, Fazal L, et al. Aldosterone inhibits the fetal program and increases hypertrophy in the heart of hypertensive mice. PLoS One. 2012;7(5): e38197. https://doi.org/10.1371/journal.pone.0038197.
Jia Z, Wang J, Shi Q, Liu S, Wang W, Tian Y, et al. SOX6 and PDCD4 enhance cardiomyocyte apoptosis through LPS-induced miR-499 inhibition. Apoptosis. 2016;21(2):174–83. https://doi.org/10.1007/s10495-015-1201-6.
Huang L, Yang L, Ding Y, Jiang X, Xia Z, You Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle. 2020;19(3):339–53. https://doi.org/10.1080/15384101.2019.1711305.
Shi Y, Han Y, Niu L, Li J, Chen Y. MiR-499 inhibited hypoxia/reoxygenation induced cardiomyocytes injury by targeting SOX6. Biotechnol Lett. 2019;41(6–7):837–47. https://doi.org/10.1007/s10529-019-02685-3.
Fornari TA, Lanaro C, Albuquerque DM, Ferreira R, Costa FF. Featured Article: Modulation of fetal hemoglobin in hereditary persistence of fetal hemoglobin deletion type-2, compared to Sicilian deltabeta-thalassemia, by BCL11A and SOX6-targeting microRNAs. Exp Biol Med (Maywood). 2017;242(3):267–74. https://doi.org/10.1177/1535370216668052.
Saleem M, Hodgkinson CP, Xiao L, Gimenez-Bastida JA, Rasmussen ML, Foss J, et al. Sox6 as a new modulator of renin expression in the kidney. Am J Physiol Renal Physiol. 2020;318(2):F285–97. https://doi.org/10.1152/ajprenal.00095.2019.
Saleem M, Saavedra-Sánchez L, Barturen-Larrea P, Gomez JA. The transcription factor Sox6 controls renin expression during renal artery stenosis. Kidney360. 2021;2(5):842–56. https://doi.org/10.34067/kid.0002792020.
Johnson T, Gaunt TR, Newhouse SJ, Padmanabhan S, Tomaszewski M, Kumari M, et al. Blood pressure loci identified with a gene-centric array. Am J Hum Genet. 2011;89(6):688–700. https://doi.org/10.1016/j.ajhg.2011.10.013.
Lu X, Wang L, Lin X, Huang J, Charles GuC, He M, et al. Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet. 2015;24(3):865–74. https://doi.org/10.1093/hmg/ddu478.
Dong C, Beecham A, Slifer S, Wang L, Blanton SH, Wright CB, et al. Genomewide linkage and peakwide association analyses of carotid plaque in Caribbean Hispanics. Stroke. 2010;41(12):2750–6. https://doi.org/10.1161/STROKEAHA.110.596981.
Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–531. https://doi.org/10.1152/physrev.00031.2010.
Hagiwara N, Yeh M, Liu A. Sox6 is required for normal fiber type differentiation of fetal skeletal muscle in mice. Dev Dyn. 2007;236(8):2062–76. https://doi.org/10.1002/dvdy.21223.
Hagiwara N, Ma B, Ly A. Slow and fast fiber isoform gene expression is systematically altered in skeletal muscle of the Sox6 mutant, p100H. Dev Dyn. 2005;234(2):301–11. https://doi.org/10.1002/dvdy.20535.
Taglietti V, Maroli G, Cermenati S, Monteverde S, Ferrante A, Rossi G, et al. Nfix induces a switch in Sox6 transcriptional activity to regulate MyHC-I expression in fetal muscle. Cell Rep. 2016;17(9):2354–66. https://doi.org/10.1016/j.celrep.2016.10.082.
Jackson HE, Ono Y, Wang X, Elworthy S, Cunliffe VT, Ingham PW. The role of Sox6 in zebrafish muscle fiber type specification. Skelet Muscle. 2015;5(1):2. https://doi.org/10.1186/s13395-014-0026-2.
Lin S, Lin X, Zhang Z, Jiang M, Rao Y, Nie Q, et al. Copy number variation in SOX6 contributes to chicken muscle development. Genes (Basel). 2018;9(1). https://doi.org/10.3390/genes9010042.
Mok GF, Lozano-Velasco E, Munsterberg A. microRNAs in skeletal muscle development. Semin Cell Dev Biol. 2017;72:67–76. https://doi.org/10.1016/j.semcdb.2017.10.032.
Horak M, Novak J, Bienertova-Vasku J. Muscle-specific microRNAs in skeletal muscle development. Dev Biol. 2016;410(1):1–13. https://doi.org/10.1016/j.ydbio.2015.12.013.
Ge Y, Chen J. MicroRNAs in skeletal myogenesis. Cell Cycle. 2011;10(3):441–8. https://doi.org/10.4161/cc.10.3.14710.
Honda M, Hidaka K, Fukada SI, Sugawa R, Shirai M, Ikawa M, et al. Vestigial-like 2 contributes to normal muscle fiber type distribution in mice. Sci Rep. 2017;7(1):7168. https://doi.org/10.1038/s41598-017-07149-0.
Duran BO, Fernandez GJ, Mareco EA, Moraes LN, Salomao RA, Gutierrez de Paula T, et al. Differential microRNA expression in fast- and slow-twitch skeletal muscle of Piaractus mesopotamicus during growth. PLoS One. 2015;10(11):e0141967. https://doi.org/10.1371/journal.pone.0141967.
Nachtigall PG, Dias MC, Carvalho RF, Martins C, Pinhal D. MicroRNA-499 expression distinctively correlates to target genes sox6 and rod1 profiles to resolve the skeletal muscle phenotype in Nile tilapia. PLoS One. 2015;10(3): e0119804. https://doi.org/10.1371/journal.pone.0119804.
Wu L, Ran L, Lang H, Zhou M, Yu L, Yi L, et al. Myricetin improves endurance capacity by inducing muscle fiber type conversion via miR-499. Nutr Metab (Lond). 2019;16:27. https://doi.org/10.1186/s12986-019-0353-8.
Huang S, Jin L, Shen J, Shang P, Jiang X, Wang X. Electrical stimulation influences chronic intermittent hypoxia-hypercapnia induction of muscle fibre transformation by regulating the microRNA/Sox6 pathway. Sci Rep. 2016;6:26415. https://doi.org/10.1038/srep26415.
Liu Y, Zhang M, Shan Y, Ji G, Ju X, Tu Y, et al. miRNA-mRNA network regulation in the skeletal muscle fiber phenotype of chickens revealed by integrated analysis of miRNAome and transcriptome. Sci Rep. 2020;10(1):10619. https://doi.org/10.1038/s41598-020-67482-9.
Wang XY, Chen XL, Huang ZQ, Chen DW, Yu B, He J, et al. MicroRNA-499-5p regulates porcine myofiber specification by controlling Sox6 expression. Animal. 2017;11(12):2268–74. https://doi.org/10.1017/S1751731117001008.
Zhang Y, Yu B, Yu J, Zheng P, Huang Z, Luo Y, et al. Butyrate promotes slow-twitch myofiber formation and mitochondrial biogenesis in finishing pigs via inducing specific microRNAs and PGC-1alpha expression1. J Anim Sci. 2019;97(8):3180–92. https://doi.org/10.1093/jas/skz187.
Zhang R, Grosse-Brinkhaus C, Heidt H, Uddin MJ, Cinar MU, Tesfaye D, et al. Polymorphisms and expression analysis of SOX-6 in relation to porcine growth, carcass, and meat quality traits. Meat Sci. 2015;107:26–32. https://doi.org/10.1016/j.meatsci.2015.04.007.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. https://doi.org/10.1016/j.diabres.2018.02.023.
Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–55. https://doi.org/10.1007/s00125-014-3462-y.
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. https://doi.org/10.1016/S0140-6736(13)60591-7.
Eliasson L, Regazzi R. Micro(RNA) Management and mismanagement of the islet. J Mol Biol. 2020;432(5):1419–28. https://doi.org/10.1016/j.jmb.2019.09.017.
Ventriglia G, Nigi L, Sebastiani G, Dotta F. MicroRNAs: novel players in the dialogue between pancreatic islets and immune system in autoimmune diabetes. Biomed Res Int. 2015;2015: 749734. https://doi.org/10.1155/2015/749734.
•• Kaur P, Kotru S, Singh S, Behera BS, Munshi A. Role of miRNAs in the pathogenesis of T2DM, insulin secretion, insulin resistance, and beta cell dysfunction: the story so far. J Physiol Biochem. 2020;76(4):485–502. https://doi.org/10.1007/s13105-020-00760-2. An extensive review on how multiple miRNAs regulate pancreatic beta-cell function and insulin resistance, hence how they participate in the pathogenesis of diabetes and other metabolic disorders.
Landrier JF, Derghal A, Mounien L. MicroRNAs in obesity and related metabolic disorders. Cells. 2019;8(8). https://doi.org/10.3390/cells8080859.
Zhang HN, Xu QQ, Thakur A, Alfred MO, Chakraborty M, Ghosh A, et al. Endothelial dysfunction in diabetes and hypertension: role of microRNAs and long non-coding RNAs. Life Sci. 2018;213:258–68. https://doi.org/10.1016/j.lfs.2018.10.028.
Miao C, Chang J, Zhang G, Fang Y. MicroRNAs in type 1 diabetes: new research progress and potential directions. Biochem Cell Biol. 2018;96(5):498–506. https://doi.org/10.1139/bcb-2018-0027.
Kaviani M, Azarpira N, Karimi MH, Al-Abdullah I. The role of microRNAs in islet beta-cell development. Cell Biol Int. 2016;40(12):1248–55. https://doi.org/10.1002/cbin.10691.
Kaspi H, Pasvolsky R, Hornstein E. Could microRNAs contribute to the maintenance of beta cell identity? Trends Endocrinol Metab. 2014;25(6):285–92. https://doi.org/10.1016/j.tem.2014.01.003.
Hamar P. Role of regulatory micro RNAs in type 2 diabetes mellitus-related inflammation. Nucleic Acid Ther. 2012;22(5):289–94. https://doi.org/10.1089/nat.2012.0381.
Iguchi H, Urashima Y, Inagaki Y, Ikeda Y, Okamura M, Tanaka T, et al. SOX6 suppresses cyclin D1 promoter activity by interacting with beta-catenin and histone deacetylase 1, and its down-regulation induces pancreatic beta-cell proliferation. J Biol Chem. 2007;282(26):19052–61. https://doi.org/10.1074/jbc.M700460200.
Iguchi H, Sakai J. SOX6 attenuates glucose-stimulated insulin secretion by repressing PDX1 transcriptional activity. Nihon Yakurigaku Zasshi. 2006;128(4):219–24. https://doi.org/10.1254/fpj.128.219.
• Melkman-Zehavi T, Oren R, Kredo-Russo S, Shapira T, Mandelbaum AD, Rivkin N, et al. miRNAs control insulin content in pancreatic beta-cells via downregulation of transcriptional repressors. EMBO J. 2011;30(5):835–45. https://doi.org/10.1038/emboj.2010.361. This pioneering study showed that several miRNAs are involved in insulin synthesis and regulation in mice. Further, they revealed that miRNA-dependent regulation of insulin expression is associated with upregulation of transcriptional repressor Sox6.
Bai C, Gao Y, Li X, Wang K, Xiong H, Shan Z, et al. MicroRNAs can effectively induce formation of insulin-producing cells from mesenchymal stem cells. J Tissue Eng Regen Med. 2017;11(12):3457–68. https://doi.org/10.1002/term.2259.
• Li Y, Deng S, Peng J, Wang X, Essandoh K, Mu X, et al. MicroRNA-223 is essential for maintaining functional beta-cell mass during diabetes through inhibiting both FOXO1 and SOX6 pathways. J Biol Chem. 2019;294(27):10438–48. https://doi.org/10.1074/jbc.RA119.007755. Using diabetic mice, they showed that miR-223 inhibits both forkhead box O1 (FOXO1) and SRY-box 6 (SOX6) signaling and modulates expression of several β-cell markers (pancreatic and duodenal homeobox 1 (PDX1), NK6 homeobox 1 (NKX6.1), and urocortin 3 (UCN3)) and cell cycle-related genes (cyclin D1, cyclin E1, and cyclin-dependent kinase inhibitor P27 (P27)) and therefore maintain the integrity and mass of beta cell mass and function.
Bai C, Li X, Gao Y, Wang K, Fan Y, Zhang S, et al. Role of microRNA-21 in the formation of insulin-producing cells from pancreatic progenitor cells. Biochim Biophys Acta. 2016;1859(2):280–93. https://doi.org/10.1016/j.bbagrm.2015.12.001.
Martinez-Sanchez A, Nguyen-Tu MS, Rutter GA. DICER inactivation identifies pancreatic beta-cell “disallowed” genes targeted by microRNAs. Mol Endocrinol. 2015;29(7):1067–79. https://doi.org/10.1210/me.2015-1059.
Gai HY, Wu C, Zhang Y, Wang D. Long non-coding RNA CHRF modulates the progression of cerebral ischemia/reperfusion injury via miR-126/SOX6 signaling pathway. Biochem Biophys Res Commun. 2019;514(2):550–7. https://doi.org/10.1016/j.bbrc.2019.04.161.
Liu S, Ren C, Qu X, Wu X, Dong F, Chand YK, et al. miR-219 attenuates demyelination in cuprizone-induced demyelinated mice by regulating monocarboxylate transporter 1. Eur J Neurosci. 2017;45(2):249–59. https://doi.org/10.1111/ejn.13485.
Zeng Z, Liu Y, Zheng W, Liu L, Yin H, Zhang S, et al. MicroRNA-129-5p alleviates nerve injury and inflammatory response of Alzheimer’s disease via downregulating SOX6. Cell Cycle. 2019;18(22):3095–110. https://doi.org/10.1080/15384101.2019.1669388.
Zhang L, Xue Z, Yan J, Wang J, Liu Q, Jiang H. LncRNA Riken-201 and Riken-203 modulates neural development by regulating the Sox6 through sequestering miRNAs. Cell Prolif. 2019;52(3): e12573. https://doi.org/10.1111/cpr.12573.
Chen J, Wu X. MicroRNA-103 contributes to osteoarthritis development by targeting Sox6. Biomed Pharmacother. 2019;118: 109186. https://doi.org/10.1016/j.biopha.2019.109186.
Georgieva VS, Etich J, Bluhm B, Zhu M, Frie C, Wilson R, et al. Ablation of the miRNA cluster 24 has profound effects on extracellular matrix protein abundance in cartilage. Int J Mol Sci. 2020;21(11). https://doi.org/10.3390/ijms21114112.
Yamashita S, Miyaki S, Kato Y, Yokoyama S, Sato T, Barrionuevo F, et al. L-Sox5 and Sox6 proteins enhance chondrogenic miR-140 microRNA expression by strengthening dimeric Sox9 activity. J Biol Chem. 2012;287(26):22206–15. https://doi.org/10.1074/jbc.M112.343194.
McIver SC, Roman SD, Nixon B, McLaughlin EA. miRNA and mammalian male germ cells. Hum Reprod Update. 2012;18(1):44–59. https://doi.org/10.1093/humupd/dmr041.
Yan N, Lu Y, Sun H, Tao D, Zhang S, Liu W, et al. A microarray for microRNA profiling in mouse testis tissues. Reproduction. 2007;134(1):73–9. https://doi.org/10.1530/REP-07-0056.
Zhang D, Li Y, Tian J, Zhang H, Wang S. MiR-202 promotes endometriosis by regulating SOX6 expression. Int J Clin Exp Med. 2015;8(10):17757–64.
Liew WC, Sundaram GM, Quah S, Lum GG, Tan JSL, Ramalingam R, et al. Belinostat resolves skin barrier defects in atopic dermatitis by targeting the dysregulated miR-335:SOX6 axis. J Allergy Clin Immunol. 2020;146(3):606–20 e12. https://doi.org/10.1016/j.jaci.2020.02.007.
Yu Y, Wang Z, Sun D, Zhou X, Wei X, Hou W, et al. miR-671 promotes prostate cancer cell proliferation by targeting tumor suppressor SOX6. Eur J Pharmacol. 2018;823:65–71. https://doi.org/10.1016/j.ejphar.2018.01.016.
Li Z, Wang Y. miR-96 targets SOX6 and promotes proliferation, migration, and invasion of hepatocellular carcinoma. Biochem Cell Biol. 2018;96(3):365–71. https://doi.org/10.1139/bcb-2017-0183.
Li YC, Li CF, Chen LB, Li DD, Yang L, Jin JP, et al. MicroRNA-766 targeting regulation of SOX6 expression promoted cell proliferation of human colorectal cancer. Onco Targets Ther. 2015;8:2981–8. https://doi.org/10.2147/OTT.S89459.
Li H, Zheng D, Zhang B, Liu L, Ou J, Chen W, et al. Mir-208 promotes cell proliferation by repressing SOX6 expression in human esophageal squamous cell carcinoma. J Transl Med. 2014;12:196. https://doi.org/10.1186/1479-5876-12-196.
Jin RH, Yu DJ, Zhong M. MiR-1269a acts as an onco-miRNA in non-small cell lung cancer via down-regulating SOX6. Eur Rev Med Pharmacol Sci. 2018;22(15):4888–97. https://doi.org/10.26355/eurrev_201808_15625.
Dang Y, Liu T, Yan J, Reinhardt JD, Yin C, Ye F, et al. Gastric cancer proliferation and invasion is reduced by macrocalyxin C via activation of the miR-212-3p/Sox6 Pathway. Cell Signal. 2020;66: 109430. https://doi.org/10.1016/j.cellsig.2019.109430.
Chen Y, Song Y, Mi Y, Jin H, Cao J, Li H, et al. microRNA-499a promotes the progression and chemoresistance of cervical cancer cells by targeting SOX6. Apoptosis. 2020;25(3–4):205–16. https://doi.org/10.1007/s10495-019-01588-y.
Acknowledgements
Graphics were produced using Biorender.com.
Funding
This work was supported by National Institutes of Health grants K01HL130497, R03HL155041, and R01HL144941 to AK.
Author information
Authors and Affiliations
Contributions
M.S. and S.R. wrote the initial draft of the manuscript. F.E. and C.L.L edited the manuscript and figures. L.A.E. and and S.K.M. prepared the figures, edited, and improved the manuscript. A.K. edited and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare that they do not have any conflict of interest/competing interests except Dr. Annet Kirabo. Dr. Kirabo has a patent entitled “Methods for Treating Inflammation and Hypertension with Gamma-Ketoaldehyde Scavengers (U.S. Patent # 14/232,615). Dr. Kirabo is an associate editor for circulation research with compensation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Mechanisms of Hypertension and Target-Organ Damage
Rights and permissions
About this article
Cite this article
Saleem, M., Rahman, S., Elijovich, F. et al. Sox6, A Potential Target for MicroRNAs in Cardiometabolic Disease. Curr Hypertens Rep 24, 145–156 (2022). https://doi.org/10.1007/s11906-022-01175-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-022-01175-8