Abstract
Purpose of Review
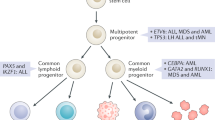
Advances in the understanding of germline predisposition to pediatric cancers, particularly myeloid neoplasms, have increased rapidly over the last 20 years. Here, we highlight the most up-to-date knowledge regarding known pathogenic germline variants that contribute to the development of myeloid neoplasms in children.
Recent Findings
This discussion enumerates the most notable myeloid neoplasm-causing germline mutations. These mutations may be organized based on their molecular underpinnings—transcriptional control, splicing and signal transduction control, and a group of heterogeneous bone marrow failure syndromes. We review recent findings related to the biochemical mechanisms that predispose to malignant transformation in each condition. Key genetic discoveries such as novel mutations, degrees of penetrance, principles of the two-hit hypothesis, and co-occurrence of multiple mutations are shared. Clinical pearls, such as information regarding epidemiology, natural history, or prognosis, are also discussed.
Summary
Germline mutations predisposing to pediatric myeloid neoplasms are frequent, but underrecognized. They hold major clinical implications regarding prognosis, treatment strategies, and screening for other malignancies. Further research is warranted to better characterize each of these conditions, as well as identify additional novel germline pathogenic variants of interest.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zhang J, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373:2336–46.
Böhles H. Die perniziöse Fanconi-Anämie, 1927. In: Historische Fälle aus der Medizin. Springer, Berlin, Heidelberg. (2020). https://doi.org/10.1007/978-3-662-59833-7_27.
Smith MA, Ries LA, Gurney JG, et al. Leukemia. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. National Cancer Institute, SEER Program, 1999;NIH 17–34.
Samaraweera SE, Wang PPS, Li KL, Casolari DA, Feng J, Pinese M, Maung KZY, Leo P, Cowley M, Perkins K, Smith AM, Ellis J, Wee A, Hiwase DK, Scott HS, Schreiber AW, Brown AL, Deans AJ, Ross DM, Moore AS, Gonda TJ, Hahn CN, D’Andrea RJ. Childhood acute myeloid leukemia shows a high level of germline predisposition. Blood. 2021;138(22):2293–8. https://doi.org/10.1182/blood.2021012666.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. https://doi.org/10.1182/blood-2016-03-643544.
Desai AV, Perpich M, Godley LA. Clinical assessment and diagnosis of germline predisposition to hematopoietic malignancies: the University of Chicago Experience. Front Pediatr. 2017;6(5):252. https://doi.org/10.3389/fped.2017.00252.
Smith ML, Cavenagh JD, Lister TA, Fitzgibbon J. Mutation of CEBPA in familial acute myeloid leukemia. N Engl J Med. 2004;351(23):2403–7. https://doi.org/10.1056/NEJMoa041331.
Mannelli F, Ponziani V, Bencini S, Bonetti MI, Benelli M, Cutini I, Gianfaldoni G, Scappini B, Pancani F, Piccini M, Rondelli T, Caporale R, Gelli AM, Peruzzi B, Chiarini M, Borlenghi E, Spinelli O, Giupponi D, Zanghì P, Bassan R, Bosi A. CEBPA-double-mutated acute myeloid leukemia displays a unique phenotypic profile: a reliable screening method and insight into biological features. Haematologica. 2017;102(3):529–40. https://doi.org/10.3324/haematol.2016.151910.
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003 Dec 15;21(24):4642–9. https://doi.org/10.1200/JCO.2003.04.036. Erratum in: J Clin Oncol. 2004;Feb 1;22(3):576. LoCocco, Francesco [corrected to Lo-Coco, Francesco].
Boddu P, Kantarjian HM, Garcia-Manero G, Ravandi F, Verstovsek S, Jabbour E, Borthakur G, Konopleva M, Bhalla KN, Daver N, DiNardo CD, Benton CB, Takahashi K, Estrov Z, Pierce SR, Andreeff M, Cortes JE, Kadia TM. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv. 2017;1(17):1312–23. https://doi.org/10.1182/bloodadvances.2017008227.
Wurm AA, Zjablovskaja P, Kardosova M, Gerloff D, Bräuer-Hartmann D, Katzerke C, Hartmann JU, Benoukraf T, Fricke S, Hilger N, Müller AM, Bill M, Schwind S, Tenen DG, Niederwieser D, Alberich-Jorda M, Behre G. Disruption of the C/EBPα-miR-182 balance impairs granulocytic differentiation. Nat Commun. 2017;8(1):46. https://doi.org/10.1038/s41467-017-00032-6.
•• Kimura Y, Iwanaga E, Iwanaga K, et al. A regulatory element in the 3′-untranslated region of CEBPA is associated with myeloid/NK/T-cell leukemia. Eur J Haematol. 2021;106:327–39. https://doi.org/10.1111/ejh.13551. DNA methylation in the CEBPA 3′-untranslated region (UTR) is associated with myeloid/NK/T-cell leukemia and CEBPA downregulation. The CEBPA 3′-UTR has the enhancer-like activity that is positively controlled by IKZF1. The methylation testing of CEBPA 3′-UTR helps to classify myeloid/NK/T-cell leukemia.
Wen XM, Hu JB, Yang J, Qian W, Yao DM, Deng ZQ, Zhang YY, Zhu XW, Guo H, Lin J, et al. CEBPA methylation and mutation in myelodysplastic syndrome. Med Oncol. 2015;32:192. https://doi.org/10.1007/s12032-015-0605-z.
Wilhelmson AS, Porse BT. CCAAT enhancer binding protein alpha (CEBPA) biallelic acute myeloid leukaemia: cooperating lesions, molecular mechanisms and clinical relevance. Br J Haematol. 2020;190:495–507. https://doi.org/10.1111/bjh.16534.
Tawana K, Wang J, Renneville A, Bodor C, Hills R, Loveday C, Savic A, Van Delft FW, Treleaven J, Georgiades P, et al. Disease evolution and outcomes in familial AML with germline CEBPA mutations. Blood. 2015;126:1214–23. https://doi.org/10.1182/blood-2015-05-647172.
Swerdlow SH. Who classification of tumours of haematopoietic and lymphoid tissues. International Agency for Research on Cancer; 2017.
Ernst MPT, Kavelaars FG, Lowenberg B, Valk PJM, Raaijmakers M. RUNX1 germline variants in RUNX1-mutant AML: how frequent? Blood. 2021;137:1428–31. https://doi.org/10.1182/blood.2020008478.
Mendler JH, Maharry K, Radmacher MD, Mrozek K, Becker H, Metzeler KH, Schwind S, Whitman SP, Khalife J, Kohlschmidt J, et al. RUNX1 mutations are associated with poor outcome in younger and older patients with cytogenetically normal acute myeloid leukemia and with distinct gene and MicroRNA expression signatures. J Clin Oncol. 2012;30:3109–18. https://doi.org/10.1200/JCO.2011.40.6652.
Gaidzik VI, Teleanu V, Papaemmanuil E, Weber D, Paschka P, Hahn J, Wallrabenstein T, Kolbinger B, Kohne CH, Horst HA, et al. RUNX1 mutations in acute myeloid leukemia are associated with distinct clinico-pathologic and genetic features. Leukemia. 2016;30:2160–8. https://doi.org/10.1038/leu.2016.126.
Drazer MW, Kadri S, Sukhanova M, Patil SA, West AH, Feurstein S, Calderon DA, Jones MF, Weipert CM, Daugherty CK, et al. Prognostic tumor sequencing panels frequently identify germ line variants associated with hereditary hematopoietic malignancies. Blood Adv. 2018;2:146–50. https://doi.org/10.1182/bloodadvances.2017013037.
OMIM entry - * 137295 - GATA-binding protein 2; GATA2. (n.d.). Retrieved from https://www.omim.org/entry/137295
Collin M, Dickinson R, Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. Br J Haematol. 2015;169:173–87. https://doi.org/10.1111/bjh.13317.
Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, et al. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–21. https://doi.org/10.1182/blood-2013-07-515528.
•• Sahoo SS, Kozyra EJ, Wlodarski MW. Germline predisposition in myeloid neoplasms: unique genetic and clinical features of GATA2 deficiency and SAMD9/SAMD9L syndromes. Best Pract Res Clin Haematol. 2020;33(3):101197. https://doi.org/10.1016/j.beha.2020.101197. A great summary highlighting the current knowledge regarding the GATA2 and SAMD9/9L syndromes with all the key references and stakeholders.
Asou H, Matsui H, Ozaki Y, Nagamachi A, Nakamura M, Aki D, et al. Identification of a common microdeletion cluster in 7q21.3 subband among patients with myeloid leukemia and myelodysplastic syndrome. Biochem Biophys Res Commun. 2009;383:245–51.
Inaba T, Nagamachi A. Revertant somatic mosaicism as a cause of cancer. Cancer Sci. 2021;112(4):1383–9. https://doi.org/10.1111/cas.14852.
Schwartz JR, Ma J, Lamprecht T, et al. The genomic landscape of pediatric myelodysplastic syndromes. Nat Commun. 2017;8:1557. https://doi.org/10.1038/s41467-017-01590-5.
Narumi S, Amano N, Ishii T, Katsumata N, Muroya K, Adachi M, Toyoshima K, Tanaka Y, Fukuzawa R, Miyako K, et al. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet. 2016;48:792–7. https://doi.org/10.1038/ng.3569.
•• de Jesus AA, Hou Y, Brooks S, Malle L, Biancotto A, Huang Y, et al. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J Clin Invest. 2020;130(4):1669–82. A very interesting paper showing the correlation between immune-dysregulatory/autoinflammatory diseases and myeloid predisposition syndromes.
Pastor VB, Sahoo SS, Boklan J, Schwabe GC, Saribeyoglu E, Strahm B, et al. Constitutional SAMD9L mutations cause familial myelodysplastic syndrome and transient monosomy 7. Haematologica. 2018;103:427–37. https://doi.org/10.3324/haematol.2017.180778.
Davidsson J, Puschmann A, Tedgard U, Bryder D, Nilsson L, Cammenga J. SAMD9 and SAMD9L in inherited predisposition to ataxia, pancytopenia, and myeloid malignancies. Leukemia. 2018;32:1106–15. https://doi.org/10.1038/s41375-018-0074-4.
Sperling AS, Gibson CJ, Ebert BL. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nat Rev Cancer. 2017;17(1):5–19. https://doi.org/10.1038/nrc.2016.112.
Sahoo SS, Pastor VP, Panda PK, Szvetnik EK, Kozyra EJ, Voss R, et al. SAMD9 and SAMD9L germline disorders in patients Enrolled in studies of the European working group of MDS in childhood (EWOG-MDS): prevalence, outcome, phenotype and functional characterisation. Blood. 2018;132(1):613. https://doi.org/10.1182/blood-2018-99-118389.
Chen DH, Below JE, Shimamura A, Keel SB, Matsushita M, Wolff J, et al. Ataxia-pancytopenia syndrome is caused by missense mutations in SAMD9L. Am J Hum Genet. 2016;98:1146–58.
Thunstrom S, Axelsson M. Leukoencephalopathia, demyelinating peripheral neuropathy and dural ectasia explained by a not formerly described de novo mutation in the SAMD9L gene, ends 27 years of investigations - a case report. BMC Neurol. 2019;19:89.
Tesi B, Davidsson J, Voss M, Rahikkala E, Holmes TD, Chiang SC, et al. Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency. MDS and neurological symptoms Blood. 2017;129(16):2266–79.
Bluteau O, Sebert M, Leblanc T, Peffault de Latour R, Quentin S, Lainey E, et al. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood. 2018;131:717–32.
Perisa MP, Rose MJ, Varga E, Kamboj MK, Spencer JD, Bajwa RPS. A novel SAMD9 variant identified in patient with MIRAGE syndrome: further defining syndromic phenotype and review of previous cases. Pediatr Blood Canc. 2019;66:e27726.
Schwartz JR, Wang S, Ma J, Lamprecht T, Walsh M, Song G, et al. Germline SAMD9 mutation in siblings with monosomy 7 and myelodysplastic syndrome. Leukemia. 2017;31:1827–30.
Wong JC, Bryant V, Lamprecht T, Ma J, Walsh M, Schwartz J, et al. Germline SAMD9 and SAMD9L mutations are associated with extensive genetic evolution and diverse hematologic outcomes. JCI Insight 2018:3
Buonocore F, Kuhnen P, Suntharalingham JP, Del Valle I, Digweed M, Stachelscheid H, et al. Somatic mutations and progressive monosomy modify SAMD9- related phenotypes in humans. J Clin Invest. 2017;127(5):1700–13.
Niemeyer CM. Pediatric MDS including refractory cytopenia and juvenile myelomonocytic leukemia. In: th Carreras E, Dufour C, Mohty M, Kroger N, editors. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies; 2019 557–60. Cham (CH).
Molina JR, Adjei AA. The Ras/Raf/MAPK pathway. J Thorac Oncol. 2006;1(1):7–9 (PMID: 17409820).
Drosten M, Dhawahir A, Sum EY, Urosevic J, Lechuga CG, Esteban LM, Castellano E, Guerra C, Santos E, Barbacid M. Genetic analysis of Ras signalling pathways in cell proliferation, migration and survival. EMBO J. 2010;29:1091–104. https://doi.org/10.1038/emboj.2010.7.
Shapiro P. Ras-MAP kinase signaling pathways and control of cell proliferation: Relevance to cancer therapy. Crit Rev Clin Lab Sci. 2002;39:285–330. https://doi.org/10.1080/10408360290795538.
Strullu M, Caye A, Lachenaud J, Cassinat B, Gazal S, Fenneteau O, Pouvreau N, Pereira S, Baumann C, Contet A, et al. Juvenile myelomonocytic leukaemia and Noonan syndrome. J Med Genet. 2014;51:689–97. https://doi.org/10.1136/jmedgenet-2014-102611.
Perez B, Mechinaud F, Galambrun C, Ben RN, Isidor B, Philip N, Derain-Court J, Cassinat B, Lachenaud J, Kaltenbach S, et al. Germline mutations of the CBL gene define a new genetic syndrome with predisposition to juvenile myelomonocytic leukaemia. J Med Genet. 2010;47:686–91. https://doi.org/10.1136/jmg.2010.076836.
Loh ML, Sakai DS, Flotho C, Kang M, Fliegauf M, Archambeault S, Mullighan CG, Chen L, Bergstraesser E, Bueso-Ramos CE, et al. Mutations in CBL occur frequently in juvenile myelomonocytic leukemia. Blood. 2009;114:1859–63. https://doi.org/10.1182/blood-2009-01-198416.
Caye A, Strullu M, Guidez F, Cassinat B, Gazal S, Fenneteau O, Lainey E, Nouri K, Nakhaei-Rad S, Dvorsky R, et al. Juvenile myelomonocytic leukemia displays mutations in components of the RAS pathway and the PRC2 network. Nat Genet. 2015;47:1334–40. https://doi.org/10.1038/ng.3420.
Stieglitz E, Taylor-Weiner AN, Chang TY, Gelston LC, Wang YD, Mazor T, Esquivel E, Yu A, Seepo S, Olsen S, et al. The genomic landscape of juvenile myelomonocytic leukemia. Nat Genet. 2015;47:1326–33. https://doi.org/10.1038/ng.3400.
Polprasert C, Schulze I, Sekeres MA, Makishima H, Przychodzen B, Hosono N, Singh J, Padgett RA, Gu X, Phillips JG, et al. Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell. 2015;27:658–70. https://doi.org/10.1016/j.ccell.2015.03.017.
Sebert M, Passet M, Raimbault A, Rahme R, Raffoux E, Sicre de Fontbrune F, Cerrano M, Quentin S, Vasquez N, Da Costa M, et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 2019;134:1441–4. https://doi.org/10.1182/blood.2019000909.
Cheah JJC, Hahn CN, Hiwase DK, et al. Myeloid neoplasms with germline DDX41 mutation. Int J Hematol. 2017;106:163–74. https://doi.org/10.1007/s12185-017-2260-y.
Lewinsohn M, Brown AL, Weinel LM, Phung C, Rafidi G, Lee MK, Schreiber AW, Feng J, Babic M, Chong CE, et al. Novel germ line DDX41 mutations define families with a lower age of MDS/AML onset and lymphoid malignancies. Blood. 2016;127:1017–23. https://doi.org/10.1182/blood-2015-10-676098.
Quesada AE, Routbort MJ, DiNardo CD, Bueso-Ramos CE, Kanagal-Shamanna R, Khoury JD, Thakral B, Zuo Z, Yin CC, Loghavi S, et al. DDX41 mutations in myeloid neoplasms are associated with male gender, TP53 mutations and high-risk disease. Am J Hematol. 2019;94:757–66.
Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, Pigneux A, Wetzler M, Stuart RK, Erba HP, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125:1367–76. https://doi.org/10.1182/blood-2014-11-610543.
Kim H, D’Andrea AD. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012;26(13):1393–408. https://doi.org/10.1101/gad.195248.112.
Lensch MW, Rathbun RK, Olson SB, Jones GR, Bagby GC Jr. Selective pressure as an essential force in molecular evolution of myeloid leukemic clones: a view from the window of Fanconi anemia. Leukemia. 1999;13:1784–9. https://doi.org/10.1038/sj.leu.2401586.
Butturini A, Gale RP, Verlander PC, Adler-Brecher B, Gillio AP, Auerbach AD. Hematologic abnormalities in Fanconi anemia: an International Fanconi Anemia Registry study. Blood. 1994;84:1650–5. https://doi.org/10.1182/blood.V84.5.1650.1650.
Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med. 2010;12(12):753–64. https://doi.org/10.1097/GIM.0b013e3181f415b5.
Kirschner M, Maurer A, Wlodarski MW, Ventura Ferreira MS, Bouillon AS, Halfmeyer I, Blau W, Kreuter M, Rosewich M, Corbacioglu S, et al. Recurrent somatic mutations are rare in patients with cryptic dyskeratosis congenita. Leukemia. 2018;32:1762–7. https://doi.org/10.1038/s41375-018-0125-x.
Danilova N, Gazda HT. Ribosomopathies: how a common root can cause a tree of pathologies. Dis Model Mech. 2015;8(9):1013–26. https://doi.org/10.1242/dmm.020529.
Aspesi A, Monteleone V, Betti M, et al. Lymphoblastoid cell lines from Diamond Blackfan anaemia patients exhibit a full ribosomal stress phenotype that is rescued by gene therapy. Sci Rep. 2017;7:12010. https://doi.org/10.1038/s41598-017-12307-5.
Zebisch A, Lal R, Muller M, Lind K, Kashofer K, Girschikofsky M, Fuchs D, Wolfler A, Geigl JB, Sill H. Acute myeloid leukemia with TP53 germ line mutations. Blood. 2016;128:2270–2. https://doi.org/10.1182/blood-2016-08-732610.
McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17:513–27. https://doi.org/10.1038/nrc.2017.60.
Mundschau G, Gurbuxani S, Gamis AS, Greene ME, Arceci RJ, Crispino JD. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood. 2003;101:4298–300.
Banno K, Omori S, Hirata K, Nawa N, Nakagawa N, Nishimura K, et al. Systematic cellular disease models reveal synergistic interaction of trisomy 21 and GATA1 mutations in hematopoietic abnormalities. Cell Rep. 2016;15:1228–41.
Lukes J, Danek P, Alejo-Valle O, et al. Chromosome 21 gain is dispensable for transient myeloproliferative disorder driven by a novel GATA1 mutation. Leukemia. 2020;34:2503–8. https://doi.org/10.1038/s41375-020-0769-1.
Hollanda LM, Lima CS, Cunha AF, Albuquerque DM, Vassallo J, Ozelo MC, et al. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet. 2006;38:807–12.
Gamis AS, Alonzo TA, Gerbing RB, Hilden JM, Sorrell AD, Sharma M, Loew TW, Arceci RJ, Barnard D, Doyle J, Massey G, Perentesis J, Ravindranath Y, Taub J, Smith FO. Natural history of transient myeloproliferative disorder clinically diagnosed in Down syndrome neonates: a report from the Children’s Oncology Group Study A2971. Blood. 2011;118(26):6752–9. https://doi.org/10.1182/blood-2011-04-350017.
Crispino JD. GATA1 mutations in Down syndrome: implications for biology and diagnosis of children with transient myeloproliferative disorder and acute megakaryoblastic leukemia. Pediatr Blood Cancer. 2005;44:40–4. https://doi.org/10.1002/pbc.20066.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Germline Predisposition to Myeloid Neoplasms
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thompson, C., Ariagno, S. & Kohorst, M.A. Pediatric Germline Predisposition to Myeloid Neoplasms. Curr Hematol Malig Rep 17, 266–274 (2022). https://doi.org/10.1007/s11899-022-00681-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-022-00681-5