Abstract
Purpose of Review
The incorporation of pegaspargase in chemotherapy regimens has significantly improved the prognosis of ALL in adults. However, pegaspargase use poses many challenges due to its unique toxicity profile. Here, we review pegaspargase’s most clinically significant toxicities, and provide guidance for their prevention and management in order to avoid unnecessary drug discontinuation and achieve maximum clinical benefit.
Recent Findings
Clinically significant toxicities of pegaspargase include thrombosis, hypersensitivity and inactivation, hepatotoxicity, pancreatitis, and hypertriglyceridemia. The majority of these toxicities are temporary, nonfatal, and can be managed supportively without permanent pegaspargase discontinuation. Special attention should be paid to inactivation, which can lead to treatment failure, as well as pancreatitis, which necessitates complete cessation of asparaginase therapy. The question of how to best proceed in patients who cannot tolerate pegaspargase remains unanswered, and is an important area of future investigation.
Summary
Pegaspargase is an essential component of the pediatric-inspired regimens that have improved survival in adult ALL. Although pegaspargase’s toxicity profile is unique, it is also highly manageable and should not be a barrier to achieving maximum clinical benefit using this drug.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012;120(6):1165–74.
Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–801.
Sive JI, Buck G, Fielding A, Lazarus HM, Litzow MR, Luger S, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157(4):463–71.
Jabbour E, O’Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121(15):2517–28.
Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2016;91(11):1645–66.
Aldoss I, Forman SJ, Pullarkat V. Acute lymphoblastic leukemia in the older adult. J Oncol Pract. 2019;15(2):67–75.
Moorman AV. The clinical relevance of chromosomal and genomic abnormalities in B-cell precursor acute lymphoblastic leukaemia. Blood Rev. 2012;26(3):123–35.
O’Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113(8):2097–101.
Rytting ME, Thomas DA, O’Brien SM, Ravandi-Kashani F, Jabbour EJ, Franklin AR, et al. Augmented Berlin-Frankfurt-Münster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL). Cancer. 2014;120(23):3660–8.
DeAngelo DJ, Stevenson KE, Dahlberg SE, Silverman LB, Couban S, Supko JG, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29(3):526–34.
• Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–59 This prospective trial incorporated more extensive use of pegaspargase, glucocorticoids, vincristine, and CNS prophylaxis in ALL patients up to 40 years old, resulting in significant improvement in EFS and OS compared to historical data.
Bussolati O, Belletti S, Uggeri J, Gatti R, Orlandini G, Dall’Asta V, et al. Characterization of apoptotic phenomena induced by treatment with L-asparaginase in NIH3T3 cells. Exp Cell Res. 1995;220(2):283–91.
Covini D, Tardito S, Bussolati O, Chiarelli LR, Pasquetto MV, Digilio R, et al. Expanding targets for a metabolic therapy of cancer: L-asparaginase. Recent Pat Anticancer Drug Discov. 2012;7(1):4–13.
Fu CH, Sakamoto KM. PEG-asparaginase. Expert Opin Pharmacother. 2007;8(12):1977–84.
Abshire TC, Pollock BH, Billett AL, Bradley P, Buchanan GR. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood. 2000;96(5):1709–15.
Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–8.
Kurtzberg J, Asselin B, Bernstein M, Buchanan GR, Pollock BH, Camitta BM. Polyethylene glycol-conjugated L-asparaginase versus native L-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: a Children’s Oncology Group Study (POG 8866). J Pediatr Hematol Oncol. 2011;33(8):610–6.
van der Sluis IM, Vrooman LM, Pieters R, Baruchel A, Escherich G, Goulden N, et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101(3):279–85.
Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol. 1999;457:621–9.
Heo YA, Syed YY, Keam SJ. Pegaspargase: a review in acute lymphoblastic leukaemia. Drugs. 2019;79(7):767–77.
Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist. 2007;12(8):991–8.
Li RJ, Jin R, Liu C, Cao X, Manning ML, Di XM, et al. FDA approval summary: calaspargase pegol-mknl for treatment of acute lymphoblastic leukemia in children and young adults. Clin Cancer Res. 2020;26(2):328–31.
Aldoss I, Douer D, Behrendt CE, Chaudhary P, Mohrbacher A, Vrona J, et al. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Eur J Haematol. 2016;96(4):375–80.
Couturier MA, Huguet F, Chevallier P, Suarez F, Thomas X, Escoffre-Barbe M, et al. Cerebral venous thrombosis in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma during induction chemotherapy with l-asparaginase: The GRAALL experience. Am J Hematol. 2015;90(11):986–91.
Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Mariani G, de Gaetano G, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216–22.
Underwood B, Zhao Q, Walker AR, Mims AS, Vasu S, Long M, et al. Incidence of venous thrombosis after peg-asparaginase in adolescent and young adults with acute lymphoblastic leukemia. Int J Hematol Oncol. 2020;9(3):IJH28.
van Zaane B, Nur E, Squizzato A, Gerdes VE, Büller HR, Dekkers OM, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost. 2010;8(11):2483–93.
Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia. 2013;27(3):553–9.
Mitchell LG, Andrew M, Hanna K, Abshire T, Halton J, Anderson R, et al. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) Study. Cancer. 2003;97(2):508–16.
Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Donati MB. Venous thrombotic complications in adults undergoing induction treatment for acute lymphoblastic leukemia: results from a meta-analysis. J Thromb Haemost. 2007;5(3):621–3.
Grace RF, Dahlberg SE, Neuberg D, Sallan SE, Connors JM, Neufeld EJ, et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br J Haematol. 2011;152(4):452–9.
Rank CU, Toft N, Tuckuviene R, Grell K, Nielsen OJ, Frandsen TL, et al. Thromboembolism in acute lymphoblastic leukemia: results of NOPHO ALL2008 protocol treatment in patients aged 1 to 45 years. Blood. 2018;131(22):2475–84.
Athale UH, Siciliano SA, Crowther M, Barr RD, Chan AK. Thromboembolism in children with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute protocols: effect of age and risk stratification of disease. Br J Haematol. 2005;129(6):803–10.
Tuckuviene R, Ranta S, Albertsen BK, Andersson NG, Bendtsen MD, Frisk T, et al. Prospective study of thromboembolism in 1038 children with acute lymphoblastic leukemia: a Nordic Society of Pediatric Hematology and Oncology (NOPHO) study. J Thromb Haemost. 2016;14(3):485–94.
• Zwicker JI, Wang TF, DeAngelo DJ, Lauw MN, Connors JM, Falanga A, et al. The prevention and management of asparaginase-related venous thromboembolism in adults: guidance from the SSC on Hemostasis and Malignancy of the ISTH. J Thromb Haemost. 2020;18(2):278–84 This review summarizes the most recent recommendations from the International Society of Thrombosis and Hemostasis (ISTH) on the management of asparaginase-related thromboembolism.
Aldoss I, Douer D. How I treat the toxicities of pegasparaginase in adults with acute lymphoblastic leukemia. Blood. 2020;135(13):987–95.
Stock W, Douer D, DeAngelo DJ, Arellano M, Advani A, Damon L, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk Lymphoma. 2011;52(12):2237–53.
Tufano A, Guida A, Di Minno MN, Prisco D, Cerbone AM, Di Minno G. Prevention of venous thromboembolism in medical patients with thrombocytopenia or with platelet dysfunction: a review of the literature. Semin Thromb Hemost. 2011;37(3):267–74.
Plander M, Szendrei T, Bodó I, Iványi JL. Successful treatment with rivaroxaban of an extended superficial vein thrombosis in a patient with acquired antithrombin deficiency due to peg-asparaginase treatment. Ann Hematol. 2015;94(7):1257–8.
Talamo L, Douvas M, Macik BG, Ornan D. Successful treatment with apixaban of sinus venous thrombosis due to pegylated asparaginase in a young adult with T cell acute lymphoblastic leukemia: case report and review of management. Ann Hematol. 2017;96(4):691–3.
Greiner J, Schrappe M, Claviez A, Zimmermann M, Niemeyer C, Kolb R, et al. THROMBOTECT - a randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents. Haematologica. 2019;104(4):756–65.
• Grace RF, DeAngelo DJ, Stevenson KE, Neuberg D, Sallan SE, Mourad YRA, et al. The use of prophylactic anticoagulation during induction and consolidation chemotherapy in adults with acute lymphoblastic leukemia. J Thromb Thrombolysis. 2018;45(2):306–14 This multicenter, prospective study of adult ALL patients receiving asparaginase showed prophylactic anticoagulation decreased risk of thromboembolism without increasing risk of bleeding events.
Hunault-Berger M, Chevallier P, Delain M, Bulabois CE, Bologna S, Bernard M, et al. Changes in antithrombin and fibrinogen levels during induction chemotherapy with L-asparaginase in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma. Use of supportive coagulation therapy and clinical outcome: the CAPELAL study. Haematologica. 2008;93(10):1488–94.
Farrell K, Fyfe A, Allan J, Tait RC, Leach M. An antithrombin replacement strategy during asparaginase therapy for acute lymphoblastic leukemia is associated with a reduction in thrombotic events. Leuk Lymphoma. 2016;57(11):2568–74.
Chen J, Ngo D, Aldoss I, Shayani S, Tsai NC, Pullarkat V. Antithrombin supplementation did not impact the incidence of pegylated asparaginase-induced venous thromboembolism in adults with acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60(5):1187–92.
Orvain C, Balsat M, Tavernier E, Marolleau JP, Pabst T, Chevallier P, et al. Thromboembolism prophylaxis in adult patients with acute lymphoblastic leukemia treated in the GRAALL-2005 study. Blood. 2020;136(3):328–38.
• Liu Y, Smith CA, Panetta JC, Yang W, Thompson LE, Counts JP, et al. Antibodies predict pegaspargase allergic reactions and failure of rechallenge. J Clin Oncol. 2019;37(23):2051–61 This study evaluated serum samples from ALL patients who received pegaspargase versus L-asparaginase. Presence of anti-pegaspargase antibodies was associated with increased drug clearance and drug failure upon re-challenge. The PEG component of pegaspargase was found to be the most common cause of antibody formation.
Burke MJ, Rheingold SR. Differentiating hypersensitivity versus infusion-related reactions in pediatric patients receiving intravenous asparaginase therapy for acute lymphoblastic leukemia. Leuk Lymphoma. 2017;58(3):540–51.
Cooper SL, Young DJ, Bowen CJ, Arwood NM, Poggi SG, Brown PA. Universal premedication and therapeutic drug monitoring for asparaginase-based therapy prevents infusion-associated acute adverse events and drug substitutions. Pediatr Blood Cancer. 2019;66(8):e27797.
Tong WH, Pieters R, Kaspers GJ, te Loo DM, Bierings MB, van den Bos C, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123(13):2026–33.
• Douer D, Aldoss I, Lunning MA, Burke PW, Ramezani L, Mark L, et al. Pharmacokinetics-based integration of multiple doses of intravenous pegaspargase in a pediatric regimen for adults with newly diagnosed acute lymphoblastic leukemia. J Clin Oncol. 2014;32(9):905–11.
Fernandez CA, Smith C, Yang W, Daté M, Bashford D, Larsen E, et al. HLA-DRB1*07:01 is associated with a higher risk of asparaginase allergies. Blood. 2014;124(8):1266–76.
Burke PW, Hoelzer D, Park JH, Schmiegelow K, Douer D. Managing toxicities with asparaginase-based therapies in adult ALL: summary of an ESMO Open-Cancer Horizons roundtable discussion. ESMO Open. 2020;5(5).
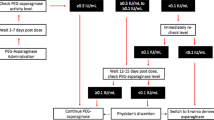
Bleyer A, Asselin BL, Koontz SE, Hunger SP. Clinical application of asparaginase activity levels following treatment with pegaspargase. Pediatr Blood Cancer. 2015;62(6):1102–5.
Salzer W, Bostrom B, Messinger Y, Perissinotti AJ, Marini B. Asparaginase activity levels and monitoring in patients with acute lymphoblastic leukemia. Leuk Lymphoma. 2018;59(8):1797–806.
• Burke PW, Aldoss I, Lunning MA, Devlin SM, Tallman MS, Pullarkat V, et al. Pegaspargase-related high-grade hepatotoxicity in a pediatric-inspired adult acute lymphoblastic leukemia regimen does not predict recurrent hepatotoxicity with subsequent doses. Leuk Res. 2018;66:49–56 This retrospective analysis of 51 adult ALL patients reported that, despite high rates of high-grade pegaspargase-associated hepatotoxicity, patients could be safely rechallenged with pegaspargase.
Sahoo S, Hart J. Histopathological features of L-asparaginase-induced liver disease. Semin Liver Dis. 2003;23(3):295–9.
Kamal N, Koh C, Samala N, Fontana RJ, Stolz A, Durazo F, et al. Asparaginase-induced hepatotoxicity: rapid development of cholestasis and hepatic steatosis. Hepatol Int. 2019;13(5):641–8.
Goekbuget N, Baumann A, Beck J, Brueggemann M, Diedrich H, Huettmann A, et al. PEG-asparaginase intensification in adult acute lymphoblastic leukemia (ALL): significant improvement of outcome with moderate increase of liver toxicity in the German Multicenter Study Group for Adult ALL (GMALL) study 07/2003. Blood. 2010;116(21):494.
Rausch CR, Marini BL, Benitez LL, Elias A, Burke PW, Bixby D, et al. PEGging down risk factors for peg-asparaginase hepatotoxicity in patients with acute lymphoblastic leukemia. Leuk Lymphoma. 2018;59(3):617–24.
Ramanujachar R, Richards S, Hann I, Goldstone A, Mitchell C, Vora A, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48(3):254–61.
Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5):1646–54.
Plourde PV, Jeha S, Hijiya N, Keller FG, Silverman LB, Rheingold SR, et al. Safety profile of asparaginase Erwinia chrysanthemi in a large compassionate-use trial. Pediatr Blood Cancer. 2014;61(7):1232–8.
Horvat TZ, Pecoraro JJ, Daley RJ, Buie LW, King AC, Rampal RK, et al. The use of Erwinia asparaginase for adult patients with acute lymphoblastic leukemia after pegaspargase intolerance. Leuk Res. 2016;50:17–20.
Oparaji JA, Rose F, Okafor D, Howard A, Turner RL, Orabi AI, et al. Risk Factors for asparaginase-associated pancreatitis: a systematic review. J Clin Gastroenterol. 2017;51(10):907–13.
Wolthers BO, Frandsen TL, Baruchel A, Attarbaschi A, Barzilai S, Colombini A, et al. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte di Legno Toxicity Working Group study. Lancet Oncol. 2017;18(9):1238–48.
Daley RJ, Rajeeve S, Kabel CC, Pappacena JJ, Stump SE, Lavery JA, et al. Tolerability and toxicity of pegaspargase in adults 40 years and older with acute lymphoblastic leukemia. Leuk Lymphoma. 2021;62(1):176–84.
Raja RA, Schmiegelow K, Sørensen DN, Frandsen TL. Asparaginase-associated pancreatitis is not predicted by hypertriglyceridemia or pancreatic enzyme levels in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2017;64(1):32–8.
Persson L, Harila-Saari A, Hed Myrberg I, Heyman M, Nilsson A, Ranta S. Hypertriglyceridemia during asparaginase treatment in children with acute lymphoblastic leukemia correlates with antithrombin activity in adolescents. Pediatr Blood Cancer. 2017;64(10).
Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–8.
Huguet F, Chevret S, Leguay T, Thomas X, Boissel N, Escoffre-Barbe M, et al. Intensified therapy of acute lymphoblastic leukemia in adults: report of the randomized GRAALL-2005 clinical trial. J Clin Oncol. 2018;36(24):2514–23.
Effer B, Lima GM, Cabarca S, Pessoa A, Farías JG, Monteiro G. L-Asparaginase from. Prep Biochem Biotechnol. 2019;49(7):679–85.
Effer B, Kleingesinds EK, Lima GM, Costa IM, Sánchez-Moguel I, Pessoa A, et al. Glycosylation of Erwinase results in active protein less recognized by antibodies. Biochem Eng J. 2020;163:107750.
Roesmann A, Afify M, Panse J, Eisert A, Steitz J, Tolba RH. L-carnitine ameliorates L-asparaginase-induced acute liver toxicity in steatotic rat livers. Chemotherapy. 2013;59(3):167–75.
Schulte RR, Madiwale MV, Flower A, Hochberg J, Burke MJ, McNeer JL, et al. Levocarnitine for asparaginase-induced hepatic injury: a multi-institutional case series and review of the literature. Leuk Lymphoma. 2018;59(10):2360–8.
Buie LW, Moore J, van Deventer H. Successful use of octreotide as a chemoprotectant for prevention of PEG-asparaginase-induced pancreatitis. Pharmacotherapy. 2014;34(8):e149–51.
Sakaguchi S, Higa T, Suzuki M, Fujimura J, Shimizu T. Prophylactic use of octreotide for asparaginase-induced acute pancreatitis. Int J Hematol. 2017;106(2):266–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
D. O. R., J. M. S., H. C. V. V. V, A. L. M., M. K. K., and F. E. C. declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Acute Lymphocytic Leukemias
Rights and permissions
About this article
Cite this article
Riley, D.O., Schlefman, J.M., Vitzthum Von Eckstaedt V, H.C. et al. Pegaspargase in Practice: Minimizing Toxicity, Maximizing Benefit. Curr Hematol Malig Rep 16, 314–324 (2021). https://doi.org/10.1007/s11899-021-00638-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00638-0