Abstract
Purpose of Review
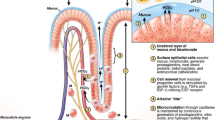
The gastroduodenal mucosal layer is a complex and dynamic system that functions in an interdependent manner to resist injury. We review and summarize the most updated knowledge about gastroduodenal defense mechanisms and specifically address (a) the mucous barrier, (b) membrane and cellular properties, and vascular, hormonal, and (c) gaseous mediators.
Recent Findings
Trefoil factor family peptides play a crucial role in cellular restitution by increasing cellular permeability and expression of aquaporin channels, aiding cellular migration and tissue repair. Additionally, evidence suggests that the symptoms of functional dyspepsia may be attributed to alterations in the duodenum, including low-grade inflammation and increased mucosal permeability.
Summary
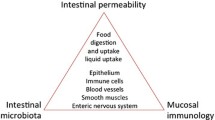
The interaction of the various mucosal protective components helps maintain structural and functional homeostasis. There is increasing evidence suggesting that the upper GI microbiota plays a crucial role in the defense mechanisms. However, this warrants further investigation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lewin MJ. Cell physiology and pharmacology of gastric acid secretion. Therapie. 1992;47(2):93–6.
Hunter J. On the digestion of the stomach after death, by John Hunter, F.R.S. and surgeon to St. George’s Hospital. Phil Trans R Soc A. 1772;62:447–54.
Pelaseyed T, Bergström JH, Gustaffson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20.
Bansil R, Turner BS. The biology of mucus: composition, synthesis and organization. Adv Drug Deliv Rev. 2018;124:3–15.
Leal J, Smyth HDC, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 2017;532(1):555–72.
Mall AS, Habte H, Mthembu Y, Peacocke J, de Beer C. Mucus and mucins: do they have a role in the inhibition of the human immunodeficiency virus? Virol J. 2017;14(1):192.
Cárdenas-Mondragón MG, Torres J, Flores-Luna L, et al. Epstein-Barr virus association with peptic ulcer disease. Anal Cell Pathol (Amst). 2015;2015:164840.
Lewis OL, Keener JP, Fogelson AL. A physics-based model for maintenance of the pH gradient in the gastric mucus layer. Am J Physiol Gastrointest Liver Physiol. 2017;313(6):G599–612.
Lock JY, Carlson TL, Carrier RL. Mucus models to evaluate the diffusion of drugs and particles. Adv Drug Deliv Rev. 2018;124:34–49.
Boccellato F, Woelffling S, Imai-Matsushima A, et al. Polarised epithelial monolayers of the gastric mucosa reveal insights into mucosal homeostasis and defence against infection. Gut. 2018.
Yandrapu H, Sarosiek J. Protective factors of the gastric and duodenal mucosa: an overview. Curr Gastroenterol Rep. 2015;17(6):24.
Caron TJ, Scott KE, Fox JG, et al. Tight junction disruption: helicobacter pylori and dysregulation of the gastric mucosal barrier. World J Gastroenterol. 2015;21(40):11411–27.
Balda MS, Matter K. Tight junctions as regulators of tissue remodelling. Curr Opin Cell Biol. 2016;42:94–101.
Li T, Liu X, Riederer B, Nikolovska K, Singh AK, Mäkelä KA, et al. Genetic ablation of carbonic anhydrase IX disrupts gastric barrier function via claudin-18 downregulation and acid backflux. Acta Physiol (Oxf). 2018;222(4):e12923.
Aihara E, Medina-Candelaria NM, Hanyu H, et al. Cell injury triggers actin polymerization to initiate epithelial restitution. J Cell Sci. 2018;131(16):jcs216317.
Sáenz JB, Mills JC. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat Rev Gastroenterol Hepatol. 2018;15(5):257–73.
Chen X, Hu Y, Xie Y, Wang Y. High salt diet can down-regulate TFF2 expression level in gastric mucosa of MGs after H. pylori infection. Microb Pathog. 2018;118:316–21.
• Marchbank T, Playford RJ. Trefoil factor family peptides enhance cell migration by increasing cellular osmotic permeability and aquaporin 3 levels. FASEB J. 2018;32(2):1017–24 Trefoil factor family peptides play a crucial role in cellular restitution by increasing cellular permeability and expression of aquaporin channels, aiding in the formation of the lamellipodium, cellular migration, and tissue repair .
Saxena B, Singh S. Comparison of three acute stress models for simulating the pathophysiology of stress-related mucosal disease. Drug Discov Ther. 2017;11(2):98–103.
Farrugia G, Szurszewski JH. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology. 2014;147(2):303–13.
Shore R, Björne H, Omoto Y, Siemiatkowska A, Gustafsson JÅ, Lindblad M, et al. Sex differences and effects of oestrogen in rat gastric mucosal defence. World J Gastroenterol. 2017;23(3):426–36.
Yoon G, Kim HS. Gastric acid response to acute exposure to hypergravity. Oncotarget. 2017;8(1):64–9.
Wallace JL, Ianaro A, de Nucci G. Gaseous mediators in gastrointestinal mucosal defense and injury. Dig Dis Sci. 2017;62(9):2223–30.
• Magierowska K, Wojcik D, Chmura A, Bakalarz D, Wierdak M, Kwiecien S, et al. Alterations in gastric mucosal expression of calcitonin gene-related peptides, vanilloid receptors, and heme oxygenase-1 mediate gastroprotective action of carbon monoxide against ethanol-induced gastric mucosal lesions. Int J Mol Sci. 2018;19(10):E2960 Carbon monoxide protects against ethanol-associated mucosal injury by increasing gastric microcirculation through the activation of transient receptor potential vanilloid receptor type 1 (located on afferent sensory fiber endings) and calcitonin gene-related peptide.
Ribeiro AR, Diniz PB, Pinheiro MS, et al. Gastroprotective effects of thymol on acute and chronic ulcers in rats: the role of prostaglandins, ATP-sensitive K(+) channels, and gastric mucus secretion. Chem Biol Interact. 2016;244:121–8.
Magierowski M, Magierowska K, Kwiecien S, Brzozowski T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules. 2015;20(5):9099–123.
Magierowski M, Magierowska K, Hubalewska-Mazgaj M, et al. Exogenous and endogenous hydrogen sulfide protects gastric mucosa against the formation and time-dependent development of ischemia/reperfusion-induced acute lesions progressing into deeper ulcerations. Molecules. 2017;22(2):E295.
Magierowski M, Jasnos K, Kwiecien S, Drozdowicz D, Surmiak M, Strzalka M, et al. Endogenous prostaglandins and afferent sensory nerves in gastroprotective effect of hydrogen sulfide against stress-induced gastric lesions. PLoS One. 2015;10(3):e0118972.
Kwiecien S, Magierowska K, Magierowski M, Surmiak M, Hubalewska-Mazgaj M, Pajdo R, et al. Role of sensory afferent nerves, lipid peroxidation and antioxidative enzymes in the carbon monoxide-induced gastroprotection against stress ulcerogenesis. J Physiol Pharmacol. 2016;67(5):717–29.
Magierowski M, Magierowska K, Hubalewska-Mazgaj M, Surmiak M, Sliwowski Z, Wierdak M, et al. Cross-talk between hydrogen sulfide and carbon monoxide in the mechanism of experimental gastric ulcers healing, regulation of gastric blood flow and accompanying inflammation. Biochem Pharmacol. 2018;149:131–42.
Alese MO, Adewole SO, Akinwunmi KF, et al. Aspirin-induced gastric lesions alters EGFR and PECAM-1 immunoreactivity in Wistar rats: modulatory action of flavonoid fraction of Musa paradisiaca. Open Access Maced J Med Sci. 2017;5(5):569–77.
Tarnawski AS, Ahluwalia A, Jones MK, et al. Expression of nerve growth factor in rat stomach. Implications for interactions between endothelial, neural and epithelial cells. J Physiol Pharmacol. 2016;67(6):879–83.
Hassan MKA, Aziz NM, Shaaban MAE, et al. Possible contribution of nitric oxide and prostaglandin in the protective effect of angiotensin (1-7) against stress induced gastric ulceration in adult male albino rats. Bratisl Lek Listy. 2016;117(12):715–21.
• Pawlik MW, Kwiecien S, Ptak-Belowska A, Pajdo R, Olszanecki R, Suski M, et al. The renin-angiotensin system and its vasoactive metabolite angiotensin-(1-7) in the mechanism of the healing of preexisting gastric ulcers. The involvement of Mas receptors, nitric oxide, prostaglandins and proinflammatory cytokines. J Physiol Pharmacol. 2016;67(1):75–91 Inhibition of angiotensin-converting enzyme and the blockade of angiotensin AT-1 receptor protects against gastric mucosal injury. Whether the metabolite of the renin-angiotensin system, Ang (1-7), accelerates the healing of already existing gastric ulcers remains to be elucidated.
Namulema J, Nansunga M, Kato CD, Kalange M, Olaleye SB. Thyroid hormones increase stomach goblet cell numbers and mucin expression during indomethacin induced ulcer healing in Wistar rats. Thyroid Res. 2018;11:6.
Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–9.
Wang B, Yao M, Longxian L, et al. The human microbiota in health and disease. Engineering. 2017;3(1):71–82.
Pereira V, Abraham P, Nallapeta S, Shetty A. Gastric bacterial flora in patients harbouring Helicobacter pylori with or without chronic dyspepsia: analysis with matrix-assisted laser desorption ionization time-of-flight mass spectroscopy. BMC Gastroenterol. 2018;18(1):20.
Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150(6):1257–61.
• Miwa H, Oshima T, Tomita T, et al. Recent understanding of the pathophysiology of functional dyspepsia: role of the duodenum as the pathogenic center. J Gastroenterol. 2019. https://doi.org/10.1007/s00535-019-01550-4 The involvement of psychological factors, diet, and H. pylori has been debated as causative factors for the pathophysiology of functional dyspepsia. New evidence suggests that the symptoms of functional dyspepsia may be brought on by alterations in the duodenum, including low-grade inflammation and increased mucosal permeability.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stomach and Duodenum
Rights and permissions
About this article
Cite this article
Galura, G.M., Chavez, L.O., Robles, A. et al. Gastroduodenal Injury: Role of Protective Factors. Curr Gastroenterol Rep 21, 34 (2019). https://doi.org/10.1007/s11894-019-0701-x
Published:
DOI: https://doi.org/10.1007/s11894-019-0701-x