Abstract
Purpose of Review
The article aims to investigate the complex relationship between cancer and cardiovascular disease (CVD), with a focus on the effects of cancer treatment on cardiac health.
Recent Findings
Advances in cancer treatment have improved long-term survival rates, but CVD has emerged as a leading cause of morbidity and mortality in cancer patients. The interplay between cancer itself, treatment methods, homeostatic changes, and lifestyle modifications contributes to this comorbidity. Recent research in the field of cardio-oncology has revealed common genetic mutations, risk factors, and metabolic features associated with the co-occurrence of cancer and CVD.
Summary
This article provides a comprehensive review of the latest research in cardio-oncology, including common genetic mutations, risk factors, and metabolic features, and explores the interactions between cancer treatment and CVD drugs, proposing novel approaches for the management of cancer and CVD.
Similar content being viewed by others
Introduction
Cancer is the disease with the highest morbidity and mortality rate in the world [1]. The development of oncology therapies has improved long-term survival rates for cancers, such as breast cancer, lung cancer, and colorectal cancer [2]; however, identifying and intervening in the therapeutic targets and risk factors to improve long-term survival and quality of life in cancer patients remains an important research topic. Cardiovascular disease (CVD) has become the second leading cause of long-term morbidity or mortality in cancer patients [3]. The characteristics of cancer itself, treatment modalities, changes in homeostasis, and lifestyle may lead to cancer comorbidity with CVD. The cardiovascular (CV) risk in the patients’ background can be significantly increased because of the progress of cancer, especially through anticancer treatment [4]. In early-stage breast cancer, CVD is even the leading cause of death in elder women (> 66 years) with more than 5 years of survival time, followed by cancer itself [5]. Whereas in childhood cancers, survivors have 8.2 times higher later CV mortality than the same age- and sex-matched population [6]. Therefore, cardio-oncology is proposed to study the cardiotoxicity caused by anti-cancer treatment or cancer itself.
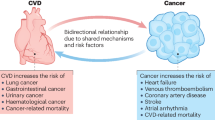
Over the past decades, cardio-oncology research mainly focused on treatment-related cardiovascular toxicity (CVT) and the additive effects on traditional CV risk factors [7••]. It has been clarified that CVT includes heart failure (HF), reduced left ventricular ejection fraction (LVEF), diastolic dysfunction, conduction disorder, arrhythmias, and arterial and venous thrombosis, especially with newer therapies [8]. However, the interaction and connection between cancer and CVD, including the overlap of risk factors and disease pathways, are calling for more attention. Of note, the study of common metabolic substrate transformation and the gene mutation spectrum provides new insight. Moreover, the application of cardiac protection is recognized as the priority task [9]. But fundamentally, the common therapeutic targets for cancer and CVD have not yet been discovered; otherwise, two diseases could be treated at one time (Fig. 1).
The shared mechanism of cancer-related heart disease. The research progress of cancer-related heart disease can be summarized in terms of common gene mutations, risk factors, and metabolic characteristics. The interaction between anticancer therapy and CVD drugs is also analyzed to facilitate the investigation of cardioprotection
Gene Mutations
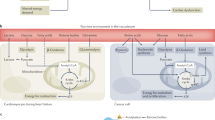
The development and progress of genomics provide an important tool for exploring the potential genetic association between cancer and CVD. In recent years, numerous studies have focused on common gene mutations in both diseases. Based on gene network analysis in a study, the most linked diseases were JAK2, TTN, and TET2, with the most closely associated diseases being perinatal cardiomyopathy, breast cancer, clonal hematopoiesis of indeterminate potential (CHIP), and coronary artery disease (CAD) [10]. Common gene mutations can be roughly classified into three pathways: inflammation, metabolism, and cell proliferation, all of which are closely related (Fig. 2).
The common genetic background of cancer and cardiovascular diseases. They can be roughly summarized into inflammation-related, metabolism-related, damage-repair, and proliferation-related pathways according to the mechanism. SCLC, small cell lung carcinoma; HGSOC, high-grade serous ovarian cancer; LDL, low-density lipoprotein; PAH-CHD, pulmonary arterial hypertension in congenital heart disease
Inflammation
In tumor microenvironment (TME), complex interactions between immune cells, inflammatory cytokines, and reactive oxygen species (ROS) activate a wide range of intracellular signaling pathways [11]. Inflammation is a key pathological feature of CVDs, such as atherosclerosis (AS) [12]. Cascade reactions lead to chronic inflammation and oxidative stress, which are the common underlying mechanisms of both diseases [13]. Macroscopically, chronic systemic inflammation, as reflected by elevated C-reactive protein (CRP) levels, is a risk factor for cancer, particularly lung cancer, in patients with stable CVD [14, 15]. The complex inflammation-related genes and pathways contain many therapeutic targets. Therefore, it is essential to understand the role of common gene mutations in the two diseases.
DNMT3A and TET2 mutations and abnormal expression in tumors and heart diseases are associated with inflammatory responses. These genes are the most frequently mutated in CHIP. There is a relationship between CVD and CHIP, and inflammation is one of the underlying mechanisms [16]. CHIP is characterized by the age-related acquisition and expansion of leukemia-causing mutations in hematopoietic stem cells (HSCs). The transformation rate of CHIP into hematologic malignancies is approximately 1% per year, and 10 to 15% of patients are over 65 years of age [17]. A retrospective cohort study showed that CHIP had a high incidence in patients with acute myocardial infarction (AMI) and cardiogenic shock (CS) and was associated with a poor prognosis [18]. Another study revealed that in patients with reduced LVEF and DNMT3A or TET2 mutations, there is an increase in HF progression (HR = 2.79; 95%CI: 1.31–5.92) and HF-related mortality or HF hospitalization (HR = 4.41; 95%CI: 2.15–9.03; p < 0.001) [19].
DNMT3A is a DNA methyltransferase that epigenetically regulates hematopoiesis and serves as a tumor suppressor gene, while TET2 encodes a tumor suppressor and transcriptional regulator that catalyzes the oxidation of 5-methylcytosine to 5-hydroxymethylcytosine in DNA [20, 21]. In protein–protein interactions, they are widely involved in chromatin organization and modification, histone modification and kinase activity regulation, and gene expression [22]. Bioinformatics analysis has indicated that CHIP-associated cpg DNA sites increase the risk of CAD by affecting lipid metabolism, immune cell recruitment, atherosclerotic plaque formation and calcification, and inflammasome production [23]. In a mouse model of chronic ischemic HF, hematopoietic or myeloid Tet2 deficiency aggravates cardiac remodeling and function while increasing IL-1β expression, while treatment with a selective NLRP3 inflammasome inhibitor prevents the development of HF [24]. In another hematopoietic cells Tet2-deficient mouse model, the size of atherosclerotic plaques was significantly increased, and researchers even suggested that targeting TET2 may be a new therapeutic target for ASCVD [21].
Recent studies have also demonstrated that the expression of DNMT3A or TET2 in macrophages is closely related to their response to interferon. Correspondingly, the reduction or deletion of these genes causes mitochondrial DNA damage, which activates the cGAS-STING pathway and promotes the activation of ISG in neighboring cells through IFNAR2 signaling, eventually leading to the occurrence of CVD [25]. Moreover, HSC division rates are increased in both mice and humans with AS. Elevated HSC division rates increase the risk of clonal hematopoiesis by 3.5-fold at age 70 in ASCVD patients, indicating that HSC proliferation is an important factor in the link between CVD and clonal hematopoiesis [26].
Apart from that, BRD4, a member of the BET (bromodomain and extra-terminal domain) protein family, participates in the expression of a variety of inflammatory gene pathways. It is closely associated with biological processes such as transcription regulation and chromatin remodeling [27]. By binding to acetylated histones, BRD4 induces the production of inflammatory factors such as tumor necrosis factor (TNF) and IL-6. BET proteins play vital roles in mammalian development and BET protein inhibitors have emerged as important anticancer agents. BRD4 links to high expression of oncogenes like MYC, NOTCH3, and NRG1 in BRD4-amplified high-grade serous ovarian cancer (HGSOC) patients [28]. BRD4-driven oncogenes promote cancer cell proliferation, genetic instability, epithelial-mesenchymal transition (EMT), metastasis, and chemotherapy resistance [29]. In small-cell lung cancer (SCLC) patients, the BRD4/ASXL3/BAP1 epigenetic axis regulates the oncogenic function of chromatin active enhancers in the SCLC-A subtype and is viewed as a novel therapeutic target [30]. In the heart, BRD4 acts as a central regulator of the profibrotic cardiac fibroblast phenotype, establishing a p38-dependent signaling pathway for epigenetic reprogramming in HF. Inhibition of BRD4 by the selective bromodomain inhibitor JQ1 also attenuated myocardial hypertrophy and HF caused by thoracic aortic coarctation [31]. Apabetalone (RVX-208), an epigenetic modulator that targets BET proteins, is also available for treatment [32].
Inflammation’s role in cancer and CVD has long been studied. Traditional inflammation-related genes, as well as IL-1B, IL-6, IL-8, and TNF-α, are involved in the pathogenesis of both diseases [33,34,35]. As the understanding of the disease deepens, more and more novel genes, including DNMT3A, TET2, BRD4, etc., are being discovered and are expected to be the therapeutic targets for comorbidities.
Metabolism
The current understanding of the genetic basis of cancer and CVD has focused on how metabolic dysregulation of cancer cells can extend beyond the TME and lead to systemic and cardiac-specific consequences. For example, cancer cells with IDH1 or IDH2 variants can produce and release increased levels of D-2-hydroxyglutarate dehydrogenase (D2-HG), while inhibition of α-ketoglutarate dehydrogenase can impair oxidative metabolism, leading to a reduction in adenosine triphosphate (ATP) supply and cardiac contractile function [36]. However, less attention has been paid to the direct effect of gene mutations on cancer and CVDs.
Mammalian dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) is a subfamily of mitogen-activated protein kinase-related protein kinases (MAPKs) that has two isoforms, DYRK1A and DYRK1B [37]. DYRK1B gene regulates the expression of the mitochondrial electron transport chain complex, oxidative phosphorylation, energy production, and mediates cancer cell proliferation and chemoresistance [38]. DYRK1B is overexpressed in cancer, maintaining cell quiescence by resisting the G0/G1-S phase transition and mediating the upregulation of antioxidant gene expression to improve cell survival [38]. Moreover, DYRK1B plays a critical role in HF. Studies have demonstrated that DYRK1B is significantly upregulated in human failing myocardium and in hypertrophic mouse hearts [39]. Mechanistically, DYRK1B positively correlates with impaired mitochondrial bioenergetics by directly binding to signal transducer and activator of transcription 3 (STAT3), increasing its phosphorylation and nuclear accumulation, ultimately leading to the downregulation of PGC-1α. Furthermore, inhibition of DYRK1B or STAT3 activity can restore cardiac function by restoring mitochondrial bioenergetics.
DYRK1A has been found to interact with TNF receptor-associated factor 3 (TRAF3) and mediate its phosphorylation, leading to the activation of the non-canonical nuclear factor kappa B (NF-κb) signaling pathway [40]. This, in turn, affects the development and occurrence of acute lymphoblastic leukemia (ALL) and offers a new concept and target for the treatment of the disease. And in the myocardial infarction (MI) mouse model, knocking down DYRK1A has been found to lead to cell cycle activation in cardiomyocytes and enhanced expression of many genes controlling cell proliferation [41]. This, along with increased H3K4me3 and H3K27ac deposition in the promoter regions of these genes, highlights the crucial role of DYRK1A in cardiac repair and suggests that it may serve as a therapeutic target for cardiomyopathy [41].
ASGR1, a tumor suppressor gene regulated by DNA methylation, is downregulated in hepatocellular carcinoma (HCC) and correlated with tumor size, grade, and survival time [42]. By promoting the binding of NLK to STAT3 and inhibiting the phosphorylation of STAT3, ASGR1 inhibits the progression of HCC both in vitro and in vivo. Interestingly, ASGR1 also functions in lipid metabolism [43]. Expressed only in hepatocytes, ASGR1-deficient mutations are associated with lower cholesterol and reduced risk of CVD [44]. This is achieved by stabilizing LXRα (liver X receptors), lowering serum and hepatic lipid levels, and blocking glycoprotein endocytosis and lysosomal degradation. Additionally, it reduces the level of amino acids in lysosomes, inhibits mammalian target of rapamycin (mTOR), and activates AMP-activated protein kinase (AMPK).
Finally, metabolic reprogramming, a hallmark of cancer and HF, is critical in optimizing nutrient utilization to meet the energy demands for proliferation, biosynthesis, and energy supply. As risk factors for cancer and CVD overlap significantly, future studies can analyze disease pathways more deeply [45].
DNA Damage Response and Cell Proliferation
DNA damage is a common feature in a range of CVD and cancer, eliciting cellular responses such as detection of damage, cell cycle arrest, DNA repair, cell senescence, and apoptosis [46•, 47]. While transient DNA damage response (DDR) from transient DNA damage is beneficial, continuously activated DDR promotes the onset and progression of the disease.
The WNT/β-catenin signaling pathway involved in DNA damage and cell proliferation is crucial in cancer and CVD, controlling gene expression in development, stem cell homeostasis, and diseases such as cancer [48]. The downstream molecule SRY-related HMG box (SOX) transcription factor is an environment-dependent regulator of WNT-reactive transcription in early cell fate decisions. SOX17 can inhibit the proliferation and tumorigenesis of cervical cancer by downregulating the activity of the WNT/β-catenin signaling pathway through β-catenin trans-inhibition [49]. In esophagus squamous cell carcinoma (ESCC), a novel SOX17low/NRF2high signature was identified, where SOX17 acts as a transcriptional repressor of NRF2, presenting a promising strategy for targeting the SOX17/NRF2 axis to overcome drug resistance [50]. The transcription factor encoded by SOX17 is also involved in the Notch signaling pathway during development and is a new risk gene for pulmonary arterial hypertension in congenital disease (PAH-CHD) and idiopathic/familial PAH [51].
Germline BRCA1/2 mutations associated with hereditary breast and ovarian cancers also associate with CVD [52]. Women with BRCA mutations had a significantly increased risk of CVD compared with women without BRCA mutations who did not undergo bilateral oophorectomy (BO) (HR = 1.82, 95%CI: 1.18–2.79) [53]. The BRCA1/2 gene regulates the repair of DNA damage, which occurs in all types of cells, including vascular endothelial cells and cancer cells [54]. At present, some studies have combined BRCA mutations with the susceptibility to myocardial injury after radiotherapy and chemotherapy, but the specific mechanism needs to be further investigated.
GRK4 is associated with both breast cancer and hypertension. In hypertension, G-protein-coupled receptor (GPCR)-mediated regulation of kidney and arterial function affects blood pressure [55]. High expression of GRK4 in breast cancer cells may activate the β-arrestins-mediated MAPK signaling pathway, suggesting that GRK4 may be involved in the development of breast cancer [56]. In addition, it is found that low expression of GRK4 in liver cancer tissues correlates with poor prognosis, and GRK4 overexpression inhibits the proliferation and migration of liver cancer cells [57].
The vast gene regulatory network has long sought to identify common regulatory targets to achieve a “multiple benefits with one hit” therapeutic effect. Genetic testing allows cancer patients to receive more targeted treatment and offers the possibility of early warning and intervention for potential CVD.
Metabolic Pathway
The transformation of metabolic patterns, specifically carbohydrate and lipid metabolism, is a common feature in cancer and CVD [58]. However, anti-cancer treatment may also lead to further metabolic disorders. Currently, research on cancer cells and CVD focuses on the relationship between obesity, hyperlipidemia, diabetes, and the occurrence and development of cancer. The molecular mechanisms of metabolic reprogramming involving glucose, fatty acids, ketone bodies, amino acids, and other substrates are being investigated (Fig. 3).
Metabolic Disorders
Metabolic disorders, particularly metabolic syndrome, represent a group of common metabolic abnormalities and disease states such as hypertension, hyperglycemia, obesity, and dyslipidemia [59]. It relates to the onset and progression of multiple chronic diseases, including cancer and CVD.
Obesity directly contributes to incident cardiovascular risk factors, including dyslipidemia, type 2 diabetes, hypertension, and sleep disorders [60]. In addition, adipose tissue secretes a variety of adipokines that affect the behavior of immune cells and tumor progression, setting the relationship between obesity and cancer [61]. However, some studies have shown that there is also an “obesity paradox” in cancer. High body mass index (BMI) does not increase the risk of progression or death in patients with early colorectal cancer [62], and may even be a protective factor [63]. This finding is consistent with the observation in patients with HF [64]. Higher BMI is associated with improved outcomes in patients with HF despite being a risk factor for the onset of HF. The possible reason is that obesity may become a metabolic reserve in cancer patients, resisting body consumption and the side effects of cachexia.
Hyperglycemia is a well-recognized risk factor for CVD, with a strong clinical relevance, and is associated with both CVD morbidity and mortality [65, 66]. The damage of endothelial, vascular smooth muscle and myocardial function caused by diabetic hyperglycemia and oxidative stress accelerates the occurrence of CVD [67]. Hyperglycemia can increase blood viscosity, aggravating the risk of thrombosis and myocardial ischemia [68]. And it is now well established that diabetes is associated with colorectal, breast, endometrial, liver, pancreatic, and bladder cancers [69]. However, the exact pathogenesis of diabetes-mediated CVD and cancer is still unclear. It has been suggested that the mechanism involves the promotion of cell proliferation by insulin-like growth factor 1 (IGF-1). IGF-1 also stimulates smooth muscle cell migration and proliferation, which is part of the pathogenesis of AS [70].
Carbohydrate Metabolism
Glucose Metabolism Reprogramming
Glucose metabolism reprogramming is reflected in the “Warburg effect” in malignant tumors, in which the pattern of cellular metabolism shifts from oxidative phosphorylation to glycolysis during nutrient deprivation [71]. In HF, early metabolism is characterized by enhanced glucose utilization and decreased fatty acid oxidation (FAO), leading to increased lactate secretion. The dependence on the ketone bodies of the heart will increase, with a reduced ability to use fatty acids [72]. However, ketone bodies are also cancer metabolites, which provide “fuel” for cancer metabolism. Ketone inhibitors have been considered new therapeutic targets, and HMGCS2, ACAT1/2, and OXCT1/2 are metabolic oncogenes [73].
Another important consequence of the “Warburg effect” is the intracellular formation of methylglyoxal (MGO), a precursor of advanced glycation end products (AGE) [74]. Cancer cell with a high glycolytic rate requires a high detoxification rate of MGO. Glyoxalase 1 encoded by the Glo1 gene acts as a rate-limiting enzyme [75]. High Glol expression may be a mechanism for the survival and growth of tumors with high rates of glycolysis and associated high flux of MGO formation [76]. Glo1 can affect glucose and lipid metabolism in spontaneously hypertensive rats (SHR) [77]. Compared with SHR wild-type rats, ShR-glo1 + / − rats had a lower epididymal fat weight and reduced ectopic fat accumulation in the liver and heart. Glo1 is also recognized as an oncogene. Its high expression links to the progression of colorectal cancer and melanoma [78, 79]. Besides, studies on glioma (GBM) have shown that Glo1 inhibition may be a potential molecular target to improve the efficacy of anticancer therapy [80].
SUMOylation: a New Player in Disease Pathology
Signal transduction, especially the kinase-related pathway, has an important role in promoting diabetes and CVD pathology [81]. SUMOylation is a hotspot in recent years. Small ubiquitin-related modifier (SUMO) is a 10-kDa post-translational modification protein [82]. Modification of the target protein can lead to conformational changes, thereby regulating protein function. Kinases such as protein kinase C (PKC), extracellular signal-regulated kinase 5 (ERK5), and 90 kDa ribosomal s6 kinases (p90RSK) are activated under diabetic conditions, being continuously modified by SUMOylation, and play a central role in regulating the signal transduction network [83]. SUMOylation also modifies insulin gene expression, glucose metabolism, and insulin exocytosis in islet β cells under physiological conditions [84].
Diabetes accelerates vascular dysfunction, leading to cardiomyopathy. Studies have found that SUMO is enhanced and X-box binding protein 1 (XBP1) nuclear translocation is impaired in a diabetic environment. Besides, Zhou et al. showed that loss of the endothelial-specific SUMO endopeptidase sentrin-specific protease 1 (SENP1) reduced pathological angiogenesis and tissue repair during hindlimb ischemia [85]. In the diabetic environment, SENP1 is downregulated, while vascular endothelial growth factor receptor 2 (VEGFR2) is hyper-SUMOylated. Expression of the non-SUMOylated form of VEGFR2 rescued the angiogenic defect in diabetic mice [85].
SUMO is also widely explored in cancers [86]. SUMOylation is elevated in pancreatic ductal adenocarcinoma (PDAC) patient samples and is a reversible post-translational modification required for cell cycle progression [87]. Pharmacological inhibition of the SUMO pathway is a potential strategy to target PDAC by inhibiting cancer cell cycle progression and activating anti-cancer immunity by inducing interferon signaling [87]. In cancer metastasis, SUMOylation is an inducer that sorts bioactive molecules into extracellular vesicles (EVs), triggers lymphangiogenesis, and then drives tumor metastasis via lymph node (LN) [88]. The EV-mediated ELNAT1/UBC9/SOX18 regulatory axis promotes lymphangiogenesis and lymph node metastasis in bladder cancer in a SUMOylation-dependent manner [89].
Lipid Metabolism
Effects of Dyslipidemia on CVD and Cancer
The direct impact of hyperlipidemia on ASCVD has been well established [90]. For cancer, studies have reported that hypercholesterolemia affects the pathogenesis of cancer by selecting cells resistant to ferroptosis [91]. Among patients with endometrial and ovarian cancer receiving endocrine therapy, there is an increased risk of CVD, of which dyslipidemia is the most common [80]. Among patients with testicular cancer, cisplatin-based radiotherapy or chemotherapy conferred an increased long-term risk for AS (HR = 4.8; 95% CI, 1.6–14.4) [92]. 27-Hydroxycholesterol (27-HC), a major metabolite of cholesterol that is also an estrogen receptor (ER) and LXR ligand, increases the incidence of ER-dependent growth and LXR-dependent metastatic breast cancer in mouse models [93]. Research on the impact of hyperglycemia on cancer and CVD is warranted.
Effects of Leptin and Adiponectin
Adipokines, especially those related to leptin and adiponectin metabolic pathways, play important roles in the process of regulation. Leptin is a 16kd adipokine produced by the obesity (OB) gene and is mainly secreted by adipose tissue [94]. Leptin at physiological concentrations can have a variety of beneficial effects to maintain glucose stability, such as binding to leptin receptor (LepR) in the hypothalamic region of the brain, acting as a neurotransmitter to convey energy status and suppressing appetite, and promoting energy expenditure [95]. Leptin is also secreted by cardiomyocytes [96], and there are leptin receptors in cardiomyocytes [97], suggesting that leptin directly regulates the metabolism of the heart to maintain normal cardiac function. Mouse models lacking cardiomyocyte leptin and its receptor result in severe CVD [98]. However, chronic elevated pathological levels or hyperleptinemia are risk factors for the development of CVD [99]. The level of circulating leptin is directly proportional to the amount of adipose tissue and is one of the indicators for the evaluation of obesity [100]. Endothelial leptin signaling is involved in cardiac fibrosis and functional deterioration by inhibiting endothelial cell autophagy and promoting endothelial dysfunction [101].
The effect of leptin has been increasingly mentioned in women’s cancer [102]. Leptin-mediated signaling through JAK/STAT3 and PI3K/AKT/mTOR pathways can increase the frequency of cancer stem cell (CSC), chemotherapy resistance, and tumor progression in breast cancer [103]. In obese mice with spontaneous breast cancer, leptin and PD-1 inhibit the glycolysis of CD8 + T cells via STAT3-driven increased FAO [104]. In HCC, berberine(BB) may play a preventive role in hepatocellular carcinoma by antagonizing the ATX-LPA-LPAR2-p38-leptin axis in the liver [105].
Cardiovascular Diseases Caused by Anti-cancer Drugs
Previous studies have explored the cardiotoxicity of anti-cancer treatments including mechanisms, biomarkers, and cardiac protection therapy. The emergence of novel drugs also brings new problems in cardiac protection. The types of diseases can be divided into cardiomyopathy, arrhythmia, vascular diseases, hypertension, valvular diseases, thrombotic disease, and others [106]. Here, we mainly focus on cardiomyopathy to find the commonality in different drugs and provide a systematic reference for cardioprotective drugs (Fig. 4).
An overview of cardiovascular toxic effects associated with cancer therapies. Many cancer therapies are associated with adverse effects and complications throughout the cardiovascular system, with some having very limited cardiovascular toxicity and others having very broad cardiovascular toxicity. HER2, human epidermal growth factor receptor 2; VEGF, vascular endothelial growth factor; mTOR, mechanistic target of rapamycin; HDAC, histone deacetylase; CDK, cyclin-dependent kinases; ICI, immune checkpoint inhibitor; CAR-T, chimeric antigen receptor T-cell therapy; AF, atrial fibrillation; SVT, supraventricular tachycardia; AV, atrioventricular; QTc, corrected QT interval
Cardiomyopathy
Cancer therapy-induced cardiomyopathy can be described as primary cardiomyopathy (direct damage to myocardial cells), secondary cardiomyopathy (perfusion changes, innervation, hormonal regulation), and myocarditis (inflammatory cell infiltration) [107]. In addition, there are still specific types of cardiomyopathies, such as cardiac amyloidosis (CA), a common injury caused by proteasome inhibitors, which has been classified as the most serious cardiotoxicity in the guidelines and needs to be closely monitored [7••].
The traditional chemotherapy drugs represented by anthracyclines are mainly primary cardiomyopathy [107]. It (1) inhibits DNA replication and RNA synthesis by intercalating between the bases of DNA double-strand; (2) inhibits topoisomerase II and hinders DNA replication and transcription; (3) produces free radicals after chelating iron ions to destroy DNA, protein, and cell membrane structure [108]. The mechanisms of anthracycline-related cardiotoxicity overlap with their anticancer effects to some extent. But studies have shown the prominent role of myocardial mitochondrial damage in impaired cardiac function [109], as ROS is the key mediator of chemotherapy-induced cardiotoxicity. Mitochondria are abundant in cardiomyocytes and are the main source of ROS [110]. At the same time, the transformation of damaged mitochondria through autophagy is essential for maintaining the structure and function of cardiomyocytes [111]. In addition, a decrease in the Bcl-2/Bax ratio, which leads to mitochondrial pore formation and activation of apoptotic pathways, also plays a role in cardiac injury [112].
Left ventricular (LV) dysfunction and HF are the common symptoms of anthracyclines-induced cardiotoxicity (AIC), most of which occur within 1 year of treatment and are considered early-onset chronic cardiotoxicity [113]. In recent years, the study of genetic susceptibility to cardiotoxicity is an emerging hotspot [114]. For example, alterations in gene expression (CYBA, GSTA1, NCF4, RAC2, ABCC1, ABCC2, and CAT) lead to mitochondrial dysfunction, resulting in increased ROS production and ultimately myocyte injury [115]. Pharmacogenomic testing has been recommended for all pediatric cancer patients with indications for treatment with DOX [116].
Cardiotoxicity caused by targeted therapies has a unique clinical phenotype. Trastuzumab, a monoclonal antibody against human epidermal growth factor receptor 2 (HER2), plays an important role in the treatment of metastatic breast cancer [117]. Trastuzumab can cause a decrease in LVEF due to the expression of ErbB2/ErbB4 receptors in cardiomyocytes [118]. NRG-1 has a protective effect on myocardial stress, but the pathway can be disrupted by HER2 antibody, leading to cardiotoxicity [119]. It is independent of the cumulative dose of trastuzumab and is generally reversible. Anthracyclines in combination with trastuzumab have shown a higher incidence of cardiotoxicity [120], possibly because of the disruption of the NRG-1 pathway. In the trial conducted by Slamon et al., concomitant administration of trastuzumab with anthracyclines provided significant clinical benefit in patients with advanced breast cancer but resulted in a high incidence of HF (28%) [121]. Clinically dose of trastuzumab significantly impaired the contractile and calcium-processing properties of induced pluripotent stem cell cardiomyocytes (iPSC-CMs) without inducing cardiomyocyte death or sarcomeric deranging [122]. RNA sequencing and functional analysis revealed that mitochondrial dysfunction and altered cardiac energy metabolism pathways were the primary causes of the trastuzumab-induced cardiotoxic phenotype.
Vascular endothelial growth factor inhibitors (VEGFi), such as bevacizumab, have been reported to cause reduced ventricular function, and even Takotsubo syndrome, which could be ascribed to its modulation of nitric oxide and catecholamine [123]. While VEGF-TKI (tyrosine kinase inhibitors) is considered to be the most closely related to cardiomyopathy among TKIs, studies in human induced pluripotent stem cell cardiomyocytes (hiPSC-CMs) suggest that insulin receptor signaling can act as a compensatory pathway in the treatment of VEGF signaling inhibition [124].
Effects of Cardiovascular Drugs on Cancers
Guideline-based HF therapy, including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB), β-receptor blocker (βRB), sodium-glucose cotransporter-2 (SGLT2) inhibitors, etc., is recommended for patients with cancer therapy-related cardiac dysfunction (CTRCD) who develop symptoms during anthracycline-based chemotherapy [7••]. While studying the cardiovascular protective effects of drugs, such drugs may also have potential effects on the tumor itself.
ACEI/ARB
ACEI/ARB/βRB has a significant cardioprotective effect in breast cancer patients with HER2-targeted therapy with reduced LVEF [125]. In a clinical trial, in 20 patients receiving HER2-targeted therapy and cardiac protection at the same time, LVEF increased gradually from an average of 49% at enrolling to 55% at 12 months (p < 0.001) [126]. However, 10% of patients may develop moderate-to-severe HF. Furthermore, different types of ACEI/ARB may bring different clinical effects. Perindopril and bisoprolol have been proven to prevent the decline in LVEF related to cancer therapy in patients with HER2-positive early breast cancer treated with trastuzumab [127]. Yet, these pharmacologic treatments did not prevent trastuzumab-mediated left ventricular remodeling.
In addition to their cardioprotective effect, ACEI drugs may also have a role in maintaining vascular function, allowing adequate delivery of chemotherapy drugs to tumor cells. In a study to explore the protective effect of zofenoprilat on DOX-induced coronary microvascular endothelial injury, endothelial cells in the exposure of DOX (0.1–1 μm) showed impaired cell survival. ERK1/2-related p53 activation, but not ROS, is responsible for DOX-induced caspase-3 cleavage. Impaired p53-mediated apoptosis was reversed by zofenoprilat treatment. In addition, the treatment of endothelium-tumor coculture with zofenoprilat did not affect the antitumor efficacy of DOX given the tumor environment. ACEI zofenoprilat has a protective effect against DOX-induced endothelial injury without affecting its antitumor efficacy.
MRA
The protective effects of mineralocorticoid receptor antagonists (MRA) or ACEI on acute and chronic doxorubicin-induced cardiotoxicity (DIC) in mice have been reported in animal models [128]. In both DIC models, DOX-induced myocardial atrophy, reduced left ventricular volume, reduced cardiomyocyte volume, and cardiac insufficiency. In the acute model, MRA and ACEI were not protective against these manifestations of DIC. In the chronic model, enalapril treatment protected against cardiac dysfunction and cardiomyocyte atrophy, and was associated with PI3K/AKT/mTOR pathway activation and normal levels of connective tissue growth factor (CTGF). MRA eplerenone was also reported to prevent left ventricular dysfunction [129]. RNA-sequencing data extracted from isolated cardiomyocytes revealed DOX’s inhibitory effect on gene expression, which was prevented by MR deletion. MRA may be suitable for the prevention of DIC. Further clinical trials may be applied for it.
βRB
Unlike ACEI, βRB may also have anti-cancer effects while protecting the heart [130]. It has been found that the sympathetic nervous system drives breast cancer progression through β-adrenergic receptor signaling pathways [131]. The effects of βRB carvedilol were explored in a mouse model of breast cancer and a large cohort of breast cancer patients (n = 4014). Carvedilol reduces primary tumor growth and metastasis in mouse models of breast cancer and prevents invasion of breast cancer cell lines through the blockage of sympathetic nervous system activation. The retrospective analysis found that women who used carvedilol at the time of diagnosis of breast cancer (n = 136) had a reduction in breast cancer-specific mortality (p = 0.076) compared with those who did not use carvedilol (n = 3878), providing a theoretical basis for exploring the use of the carvedilol as a new strategy to slow cancer progression. Another prospective, randomized, double-blind, placebo-controlled study was designed to evaluate the role of carvedilol in the prevention of DIC [132]. A total of 200 patients were enrolled, and the incidence of cardiotoxicity was 13.5–14.5%. There was no significant difference in the changes of LVEF and brain natriuretic peptide (BNP) between the two groups, the level of troponin I (TnI) in the carvedilol group was lower (p = 0.003), and the incidence of diastolic dysfunction in the carvedilol group was lower (p = 0.039).β-adrenergic receptors activate cAMP/PKA/MAPK pathways in pancreatic cancer cells, suggesting that β-receptors may play a role in cancer invasion, while β-blockers may inhibit invasion and proliferation [133]. β2 receptor antagonists significantly alter the expression of VEGF, cyclooxygenase 2 (COX-2), matrix metalloproteinase-2 (MMP-2), and MMP-9, thereby inhibiting invasion and proliferation, which may be a novel strategy for the prevention and treatment of cancer. And carvedilol has also been shown to have a protective effect against skin cancer based on promoting β-suppressor protein-mediated processes such as ERK phosphorylation [134]. Research showed that carvedilol and alprolol blocked EGF-induced phosphorylation and activation of c-Jun/AP-1 and ELK-1. Both carvedilol and alprolol effectively inhibited EGF-induced tumor transformation of JB6 P + cells.
SGLT2 Inhibitors
SGLT2 inhibitors, specifically their relevance to metabolic reprogramming, play a vital role in cardiorenal protection [135]. SGLT2 inhibitors improve outcomes in patients with HF and are protective in patients undergoing anti-cancer treatments. In a study of 3033 patients with diabetes mellitus (DM) and cancer who received anthracyclines, the group treated with SGLT2 inhibitors had a lower incidence of cardiac events than the control group (p = 0.025) and also lower overall mortality (p < 0.001), showing preliminary evidence that SGLT2 inhibitors are associated with a lower rate of cardiac events and a moderate safety profile [136]. In another study, empagliflozin (EMPA), an SGLT-2 inhibitor, improves myocardial strain and reduces myocardial fibrosis and pro-inflammatory cytokines in DOX-treated non-diabetic mice [137]. Mechanically, it improves cardiac function by participating in NLRP3 and MyD88-related pathways. EMPA also showed a protective effect against sunitinib-induced cardiac dysfunction in mice via the AMPK-mTOR signaling pathway [138]. However, these effects still need to be validated in clinical trials.
Recent evidence suggests that tumor cells also express sodium-glucose transporters in addition to classical glucose transporters [139]. Although high glucose uptake and glycolysis rates are common in tumors, the pharmacology of blocking glucose utilization in tumor therapy is not well defined. SGLT inhibitors have the potential to exert antitumor activity by restricting glucose uptake. Canagliflozin, another SGLT-2 inhibitor, can inhibit the proliferation of breast cancer cells, and its anti-proliferation effect was not affected by glucose availability and SGLT2 expression level [140]. Canagliflozin reduces oxygen consumption and glutamine metabolism through the citric acid cycle. The antiproliferative effect of canagliflozin is related to the inhibition of respiration promoting glutamine metabolism, which is an unreported mechanism for the potential antitumor effect.
Emerging Drugs and Treatments
Phosphodiesterase 5 (PDE5) inhibitors have a strong protective effect against myocardial ischemia/reperfusion injury, DIC, and improve the efficacy of stem cells in myocardial repair [141]. Meanwhile, PDE5 is highly expressed in a variety of human cancers and is thought to be involved in tumor progression [142]. Recent studies suggest that PDE5 inhibitors, as an important adjunctive drug, enhance the sensitivity of certain types of cancer to chemotherapy drugs or inhibit adverse drug reactions [143, 144]. Many clinical trials of PDE5 inhibitors have focused on potential cardiovascular and anti-cancer benefits [145]. Interestingly, it can also reduce the side effect of some anti-cancer drugs. Through high-throughput screening, PDE5 inhibitors, including sildenafil, were found to be non-toxic and effective inhibitors of romidepsin-induced EBV reactivation, with downstream cGMP/PKG pathways negatively associated with EBV reactivation in NKTL cells [146].
Calpain is a family of calcium-dependent mercaptoproteases associated with CVD [147]. Proapoptotic effects of inhibiting calpain are associated with decreased protein kinase B (AKT) protein and mRNA expression and reduced phosphorylation of glycogen synthetase kinase-3β (GSK-3β) in DOX-treated cardiomyocytes [148]. Myocardial dysfunction in calpains knock-out mice was aggravated by overexpression of calpain inhibitors, and the 5-day mortality rate in transgenic mice (29.16%) was higher than in wild-type litter mice (8%). Overexpression of calpastatin may enhance DOX-induced myocardial injury by inhibiting calpain.
Last but not least, pharmacogenomics, as an individualized approach to determining genetic differences in drug configuration and treatment response, can identify markers that predict adverse effects, enhance drug efficacy, reduce toxicity, and analyze genetic susceptibility [149]. In the presence of vincristine treatment, children who express CYP3A5 have been shown to have a significantly lower risk of peripheral neuropathy than those who express the inactive form or CYP3A4 isoform, suggesting that pretreatment genetic testing is a reliable means of risk stratification [150].
Conclusion
In summary, we review the research progress of cancer-related CVD from the aspects of common gene mutations, risk factors, and metabolic characteristics. We also analyzed the interactions between anticancer treatment and CVD drugs and proposed the benefits of novel cancer and CVD treatment. Future research directions include risk stratification of cancer patients, identifying specific patterns of early myocardial injury, serum markers, and imaging. It can also be the improvements in interventional diagnostic methods and the application of artificial intelligence (AI) analysis to identify individuals at risk of cancer and new parameters to predict risk and response to specific cardioprotective interventions.
Availability of Data and Materials
Not applicable.
Abbreviations
- AIC:
-
Anthracyclines-induced cardiotoxicity
- CAR:
-
Chimeric antigen receptor
- CHIP:
-
Clonal hematopoiesis of indeterminate potential
- CRS:
-
Cytokine release syndrome
- CTRCD:
-
Cancer therapy-related cardiac dysfunction
- CVD:
-
Cardiovascular disease
- DIC:
-
Doxorubicin-induced cardiotoxicity
- FAO:
-
Fatty acid oxidation
- HF:
-
Heart failure
- LVEF:
-
Left ventricular ejection fraction
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88.
Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–85.
Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66:309–25.
Tufano A, Coppola A, Galderisi M. The growing impact of cardiovascular oncology: epidemiology and pathophysiology. Semin Thromb Hemost. 2021;47:899–906.
Abdel-Qadir H, Austin PC, Lee DS, et al. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2:88–93.
Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–27.
••Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–361. The 2022 ESC Guidelines on cardio-oncology are an essential reference for a comprehensive review on cardio-oncology, providing up-to-date, authoritative, and multidisciplinary insights that can help shape the discussion on the management of cancer and CVD.
Guha A, Armanious M, Fradley MG. Update on cardio-oncology: novel cancer therapeutics and associated cardiotoxicities. Trends Cardiovasc Med. 2019;29:29–39.
Lenihan DJ, Fradley MG, Dent S, et al. Proceedings From the Global Cardio-Oncology Summit: The Top 10 Priorities to Actualize for CardioOncology. JACC CardioOncol. 2019;1:256–72.
Turk A, Kunej T. Shared genetic risk factors between cancer and cardiovascular diseases. Front Cardiovasc Med. 2022;9: 931917.
Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–6.
Liberale L, Badimon L, Montecucco F, Lüscher TF, Libby P, Camici GG. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. 2022;79:837–47.
Masoudkabir F, Sarrafzadegan N, Gotay C, et al. Cardiovascular disease and cancer: evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis. 2017;263:343–51.
Lüscher TF. Cardio-oncology: low-grade inflammation as a common pathway of cancer and cardiovascular disease. Eur Heart J. 2019;40:3871–4.
Abplanalp WT, Mas-Peiro S, Cremer S, John D, Dimmeler S, Zeiher AM. Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic postischemic heart failure. JAMA Cardiol. 2020;5:1170–5.
Calvillo-Argüelles O, Jaiswal S, Shlush LI, et al. Connections between clonal hematopoiesis, cardiovascular disease, and cancer: a review. JAMA Cardiol. 2019;4:380–7.
Natarajan P, Jaiswal S, Kathiresan S. Clonal hematopoiesis: somatic mutations in blood cells and atherosclerosis. Circ Genom Precis Med. 2018;11: e001926.
Böhme M, Desch S, Rosolowski M, et al. Impact of clonal hematopoiesis in patients with cardiogenic shock complicating acute myocardial infarction. J Am Coll Cardiol. 2022;80:1545–56.
Pascual-Figal DA, Bayes-Genis A, Díez-Díez M, et al. Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2021;77:1747–59.
Aavik E, Babu M, Ylä-Herttuala S. DNA methylation processes in atheosclerotic plaque. Atherosclerosis. 2019;281:168–79.
Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–7.
Senguttuvan NB, Subramanian V, Venkatesan V, Muralidharan TR, Sankaranarayanan K. Clonal hematopoiesis of indeterminate potential (CHIP) and cardiovascular diseases-an updated systematic review. J Genet Eng Biotechnol. 2021;19:105.
Uddin MDM, Nguyen NQH, Yu B, et al. Clonal hematopoiesis of indeterminate potential, DNA methylation, and risk for coronary artery disease. Nat Commun. 2022;13:5350.
Sano S, Oshima K, Wang Y, et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–86.
Cobo I, Tanaka TN, Chandra Mangalhara K, et al. DNA methyltransferase 3 alpha and TET methylcytosine dioxygenase 2 restrain mitochondrial DNA-mediated interferon signaling in macrophages. Immunity. 2022;55.
Heyde A, Rohde D, McAlpine CS, et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell. 2021;184.
Wang N, Wu R, Tang D, Kang R. The BET family in immunity and disease. Signal Transduct Target Ther. 2021;6:23.
Doroshow DB, Eder JP, LoRusso PM. BET inhibitors: a novel epigenetic approach. Ann Oncol. 2017;28:1776–87.
Drumond-Bock AL, Bieniasz M. The role of distinct BRD4 isoforms and their contribution to high-grade serous ovarian carcinoma pathogenesis. Mol Cancer. 2021;20:145.
Szczepanski AP, Zhao Z, Sosnowski T, Goo YA, Bartom ET, Wang L. ASXL3 bridges BRD4 to BAP1 complex and governs enhancer activity in small cell lung cancer. Genome Med. 2020;12:63.
Mu J, Zhang D, Tian Y, Xie Z, Zou M-H. BRD4 inhibition by JQ1 prevents high-fat diet-induced diabetic cardiomyopathy by activating PINK1/Parkin-mediated mitophagy in vivo. J Mol Cell Cardiol. 2020;149.
Ray KK, Nicholls SJ, Buhr KA, et al. Effect of apabetalone added to standard therapy on major adverse cardiovascular events in patients with recent acute coronary syndrome and type 2 diabetes: a randomized clinical trial. JAMA. 2020;323:1565–73.
Ganatra S, Dani SS, Yang EH, Zaha VG, Nohria A. Cardiotoxicity of T-cell antineoplastic therapies: JACC: CardioOncology Primer. JACC CardioOncol. 2022;4:616–23.
Tulotta C, Lefley DV, Moore CK, et al. IL-1B drives opposing responses in primary tumours and bone metastases; harnessing combination therapies to improve outcome in breast cancer. NPJ Breast Cancer. 2021;7:95.
Finke D, Heckmann MB, Frey N, Lehmann LH. Cancer-a major cardiac comorbidity with implications on cardiovascular metabolism. Front Physiol. 2021;12:729713.
Karlstaedt A, Zhang X, Vitrac H, et al. Oncometabolite d-2-hydroxyglutarate impairs α-ketoglutarate dehydrogenase and contractile function in rodent heart. Proc Natl Acad Sci U S A. 2016;113:10436–41.
Martin SG, Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature. 2009;459:852–6.
Becker W. A wake-up call to quiescent cancer cells - potential use of DYRK1B inhibitors in cancer therapy. FEBS J. 2018;285:1203–11.
Zhuang L, Jia K, Chen C, et al. DYRK1B-STAT3 drives cardiac hypertrophy and heart failure by impairing mitochondrial bioenergetics. Circulation. 2022;145:829–46.
Li Y, Xie X, Jie Z, et al. DYRK1a mediates BAFF-induced noncanonical NF-κB activation to promote autoimmunity and B-cell leukemogenesis. Blood. 2021;138:2360–71.
Lan C, Chen C, Qu S, et al. Inhibition of DYRK1A, via histone modification, promotes cardiomyocyte cell cycle activation and cardiac repair after myocardial infarction. EBioMedicine. 2022;82: 104139.
Zhu X, Song G, Zhang S, et al. Asialoglycoprotein receptor 1 functions as a tumor suppressor in liver cancer via inhibition of STAT3. Cancer Res. 2022;82:3987–4000.
Wang J-Q, Li L-L, Hu A, et al. Inhibition of ASGR1 decreases lipid levels by promoting cholesterol excretion. Nature. 2022;608:413–20.
Huynh K. Genetics: Variants in ASGR1 linked to reduced CAD risk. Nat Rev Cardiol. 2016;13:442.
Karlstaedt A, Taegtmeyer H. Cardio-onco-metabolism - metabolic vulnerabilities in cancer and the heart. J Mol Cell Cardiol. 2022;171:71–80.
•Uryga A, Gray K, Bennett M. DNA damage and repair in vascular disease. Annu Rev Physiol. 2016;78:45–66. This article reviews the relationship between cardiovascular diseases and cancers from the perspective of metabolic disorders and reprogramming, providing a reference for the prevention, diagnosis and treatment strategies of cancer-related cardiovascular diseases.
Pilié PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019;16.
Mukherjee S, Luedeke DM, McCoy L, Iwafuchi M, Zorn AM. SOX transcription factors direct TCF-independent WNT/β-catenin responsive transcription to govern cell fate in human pluripotent stem cells. Cell Rep. 2022;40: 111247.
Li L, Yang W-T, Zheng P-S, Liu X-F. SOX17 restrains proliferation and tumor formation by down-regulating activity of the Wnt/β-catenin signaling pathway via trans-suppressing β-catenin in cervical cancer. Cell Death Dis. 2018;9:741.
Hsieh C-H, Kuan W-H, Chang W-L, et al. Dysregulation of SOX17/NRF2 axis confers chemoradiotherapy resistance and emerges as a novel therapeutic target in esophageal squamous cell carcinoma. J Biomed Sci. 2022;29:90.
Zhu N, Welch CL, Wang J, et al. Rare variants in SOX17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Med. 2018;10:56.
Zbuk K, Xie C, Young R, et al. BRCA2 variants and cardiovascular disease in a multi-ethnic study. BMC Med Genet. 2012;13:56.
do Valle HA, Kaur P, Kwon JS, Cheifetz R, Dawson L, Hanley GE. Risk of cardiovascular disease among women carrying BRCA mutations after risk-reducing bilateral salpingo-oophorectomy: a population-based study. Gynecol Oncol. 2021;162:707–714.
Groelly FJ, Fawkes M, Dagg RA, Blackford AN, Tarsounas M. Targeting DNA damage response pathways in cancer. Nat Rev Cancer. 2023;23:78–94.
Yang J, Hall JE, Jose PA, Chen K, Zeng C. Comprehensive insights in GRK4 and hypertension: from mechanisms to potential therapeutics. Pharmacol Ther. 2022;239:108194.
Matsubayashi J, Takanashi M, Oikawa K, et al. Expression of G protein-coupled receptor kinase 4 is associated with breast cancer tumourigenesis. J Pathol. 2008;216:317–27.
Luo Y, Wang Z, Xiao S, Li R, Jiang X. G protein-coupled receptor kinase 4 is a novel prognostic factor in hepatocellular carcinoma. Dis Markers. 2022;2022:2628879.
Karlstaedt A, Barrett M, Hu R, Gammons ST, Ky B. Cardio-oncology: understanding the intersections between cardiac metabolism and cancer biology. JACC Basic Transl Sci. 2021;6:705–18.
Mili N, Paschou SA, Goulis DG, Dimopoulos M-A, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: pathophysiological and therapeutic associations. Endocrine. 2021;74:478–97.
Katta N, Loethen T, Lavie CJ, Alpert MA. Obesity and coronary heart disease: epidemiology, pathology, and coronary artery imaging. Curr Probl Cardiol. 2021;46:100655.
Singh A, Mayengbam SS, Yaduvanshi H, Wani MR, Bhat MK. Macrophage functionality in obesity favors cancer progression. Cancer Res. 2022.
Renfro LA, Loupakis F, Adams RA, et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol. 2016;34:144–50.
Kasi PM, Zafar SY, Grothey A. Is obesity an advantage in patients with colorectal cancer? Expert Rev Gastroenterol Hepatol. 2015;9:1339–42.
Lavie CJ, Ventura HO. The obesity paradox in heart failure: is it all about fitness, fat, or sex? JACC Heart Fail. 2015;3:927–30.
Yun J-S, Ko S-H. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism. 2021;123:154838.
Newman JD, Schwartzbard AZ, Weintraub HS, Goldberg IJ, Berger JS. Primary prevention of cardiovascular disease in diabetes mellitus. J Am Coll Cardiol. 2017;70:883–93.
Pektas MB, Turan O, Ozturk Bingol G, Sumlu E, Sadi G, Akar F. High glucose causes vascular dysfunction through Akt/eNOS pathway: reciprocal modulation by juglone and resveratrol. Can J Physiol Pharmacol. 2018;96:757–64.
Irace C, Carallo C, Scavelli F, De Franceschi MS, Esposito T, Gnasso A. Blood viscosity in subjects with normoglycemia and prediabetes. Diabetes Care. 2014;37:488–92.
Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21.
Satija A, Spiegelman D, Giovannucci E, Hu FB. Type 2 diabetes and risk of cancer. BMJ. 2015;350:g7707.
Icard P, Shulman S, Farhat D, Steyaert J-M, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38.
Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 2020;141:1800–12.
Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Ketone body utilization drives tumor growth and metastasis. Cell Cycle. 2012;11:3964–71.
Bellahcène A, Nokin M-J, Castronovo V, Schalkwijk C. Methylglyoxal-derived stress: an emerging biological factor involved in the onset and progression of cancer. Semin Cancer Biol. 2018;49:64–74.
Lee JH, Subedi L, Kim SY. Effect of cysteine on methylglyoxal-induced renal damage in mesangial cells. Cells. 2020;9.
Schalkwijk CG, Stehouwer CDA. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev. 2020;100:407–61.
Šilhavý J, Malínská H, Hüttl M, et al. Downregulation of the gene is associated with reduced adiposity and ectopic fat accumulation in spontaneously hypertensive rats. Antioxidants (Basel). 2020;9.
Sakellariou S, Fragkou P, Levidou G, et al. Clinical significance of AGE-RAGE axis in colorectal cancer: associations with glyoxalase-I, adiponectin receptor expression and prognosis. BMC Cancer. 2016;16:174.
Jandova J, Wondrak GT. Genomic GLO1 deletion modulates TXNIP expression, glucose metabolism, and redox homeostasis while accelerating human A375 malignant melanoma tumor growth. Redox Biol. 2021;39:101838.
Jandial R, Neman J, Lim PP, et al. Inhibition of GLO1 in glioblastoma multiforme increases DNA-AGEs, stimulates RAGE expression, and inhibits brain tumor growth in orthotopic mouse models. Int J Mol Sci. 2018;19.
Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62:1543–52.
Agarwal N, Taberner FJ, Rangel Rojas D, et al. SUMOylation of enzymes and ion channels in sensory neurons protects against metabolic dysfunction, neuropathy, and sensory loss in diabetes. Neuron. 2020;107.
Chang E, Abe J-I. Kinase-SUMO networks in diabetes-mediated cardiovascular disease. Metabolism. 2016;65:623–33.
Li N, Zhang S, Xiong F, Eizirik DL, Wang C-Y. SUMOylation, a multifaceted regulatory mechanism in the pancreatic beta cells. Semin Cell Dev Biol. 2020;103:51–8.
Zhou HJ, Xu Z, Wang Z, et al. SUMOylation of VEGFR2 regulates its intracellular trafficking and pathological angiogenesis. Nat Commun. 2018;9:3303.
Seeler J-S, Dejean A. SUMO and the robustness of cancer. Nat Rev Cancer. 2017;17:184–97.
Wang T, Wu J, Dong W, et al. The MEK inhibitor U0126 ameliorates diabetic cardiomyopathy by restricting XBP1’s phosphorylation dependent SUMOylation. Int J Biol Sci. 2021;17:2984–99.
Steinbichler TB, Dudás J, Riechelmann H, Skvortsova I-I. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–81.
Chen C, Zheng H, Luo Y, et al. SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J Clin Invest. 2021;131.
Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61:21–8.
Liu W, Chakraborty B, Safi R, Kazmin D, Chang C-Y, McDonnell DP. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat Commun. 2021;12:5103.
Haugnes HS, Wethal T, Aass N, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. 2010;28:4649–57.
Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–8.
Pereira S, Cline DL, Glavas MM, Covey SD, Kieffer TJ. Tissue-Specific effects of leptin on glucose and lipid metabolism. Endocr Rev. 2021;42.
Xu J, Bartolome CL, Low CS, et al. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature. 2018;556:505–9.
Fonfara S, Kitz S, Hetzel U, Kipar A. Myocardial leptin transcription in feline hypertrophic cardiomyopathy. Res Vet Sci. 2017;112:105–8.
Khokhlova A, Myachina T, Butova X, et al. The acute effects of leptin on the contractility of isolated rat atrial and ventricular cardiomyocytes. Int J Mol Sci. 2022;23.
McGaffin KR, Witham WG, Yester KA, et al. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc Res. 2011;89:60–71.
Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–10.
Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain-adipose crosstalks. Nat Rev Neurosci. 2018;19:153–65.
Gogiraju R, Hubert A, Fahrer J, et al. Endothelial leptin receptor deletion promotes cardiac autophagy and angiogenesis following pressure overload by suppressing Akt/mTOR signaling. Circ Heart Fail. 2019;12:e005622.
Tewari S, Vargas R, Reizes O. The impact of obesity and adipokines on breast and gynecologic malignancies. Ann N Y Acad Sci. 2022.
Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L, Sánchez-Margalet V. Obesity and breast cancer: role of leptin. Front Oncol. 2019;9:596.
Zhang C, Yue C, Herrmann A, et al. STAT3 activation-induced fatty acid oxidation in CD8 T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab. 2020;31.
Ren G, Guo J-H, Feng C-L, et al. Berberine inhibits carcinogenesis through antagonizing the ATX-LPA-LPAR2-p38-leptin axis in a mouse hepatoma model. Mol Ther Oncolytics. 2022;26:372–86.
Herrmann J, Lenihan D, Armenian S, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–99.
Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17:474–502.
Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104:971–7.
Galán-Arriola C, Villena-Gutiérrez R, Higuero-Verdejo MI, et al. Remote ischaemic preconditioning ameliorates anthracycline-induced cardiotoxicity and preserves mitochondrial integrity. Cardiovasc Res. 2021;117:1132–43.
Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139: 111708.
Saito T, Hamano K, Sadoshima J. Molecular mechanisms and clinical implications of multiple forms of mitophagy in the heart. Cardiovasc Res. 2021;117:2730–41.
Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res. 2002;62:4592–8.
Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–8.
Petrykey K, Andelfinger GU, Laverdière C, Sinnett D, Krajinovic M. Genetic factors in anthracycline-induced cardiotoxicity in patients treated for pediatric cancer. Expert Opin Drug Metab Toxicol. 2020;16:865–83.
Yang X, Li G, Yang T, et al. Possible susceptibility genes for intervention against chemotherapy-induced cardiotoxicity. Oxid Med Cell Longev. 2020;2020:4894625.
Chang VY, Wang JJ. Pharmacogenetics of chemotherapy-induced cardiotoxicity. Curr Oncol Rep. 2018;20:52.
Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134–50.
Dent SF, Morse A, Burnette S, Guha A, Moore H. Cardiovascular toxicity of novel HER2-targeted therapies in the treatment of breast cancer. Curr Oncol Rep. 2021;23:128.
Kurokawa YK, Shang MR, Yin RT, George SC. Modeling trastuzumab-related cardiotoxicity in vitro using human stem cell-derived cardiomyocytes. Toxicol Lett. 2018;285:74–80.
van der Voort A, van Ramshorst MS, van Werkhoven ED, et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual ERBB2 blockade in patients with ERBB2-positive breast cancer: a secondary analysis of the TRAIN-2 randomized, phase 3 trial. JAMA Oncol. 2021;7:978–84.
Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83.
Kitani T, Ong S-G, Lam CK, et al. Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation. 2019;139:2451–65.
White AJ, LaGerche A, Toner GC, Whitbourn RJ. Apical ballooning syndrome during treatment with a vascular endothelial growth factor receptor antagonist. Int J Cardiol. 2009;131:e92–4.
Lamore SD, Ahlberg E, Boyer S, et al. Deconvoluting kinase inhibitor induced cardiotoxicity. Toxicol Sci. 2017;158:213–26.
Lynce F, Barac A, Geng X, et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175:595–603.
Leong DP, Cosman T, Alhussein MM, et al. Safety of continuing trastuzumab despite mild cardiotoxicity: a phase I trial. JACC Cardio Oncol. 2019;1.
Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol. 2017;35:870–7.
Hullin R, Métrich M, Sarre A, et al. Diverging effects of enalapril or eplerenone in primary prevention against doxorubicin-induced cardiotoxicity. Cardiovasc Res. 2018;114:272–81.
Lother A, Bergemann S, Kowalski J, et al. Inhibition of the cardiac myocyte mineralocorticoid receptor ameliorates doxorubicin-induced cardiotoxicity. Cardiovasc Res. 2018;114:282–90.
Løfling LL, Støer NC, Sloan EK, et al. β-blockers and breast cancer survival by molecular subtypes: a population-based cohort study and meta-analysis. Br J Cancer. 2022;127:1086–96.
Gillis RD, Botteri E, Chang A, et al. Carvedilol blocks neural regulation of breast cancer progression in vivo and is associated with reduced breast cancer mortality in patients. Eur J Cancer. 2021;147:106–16.
Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71:2281–90.
Zhang D, Ma Q-Y, Hu H-T, Zhang M. β2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFκB and AP-1. Cancer Biol Ther. 2010;10:19–29.
Cleveland KH, Yeung S, Huang KM, Liang S, Andresen BT, Huang Y. Phosphoproteome profiling provides insight into the mechanism of action for carvedilol-mediated cancer prevention. Mol Carcinog. 2018;57.
Gao Y-M, Feng S-T, Wen Y, Tang T-T, Wang B, Liu B-C. Cardiorenal protection of SGLT2 inhibitors-perspectives from metabolic reprogramming. EBioMedicine. 2022;83:104215.
CA Gongora ZD Drobni QAC Silva T, et al. Sodium-glucose co-transporter-2 inhibitors and cardiac outcomes among patients treated with anthracyclines. JACC Heart Fail. 2022;10:559–567.
Quagliariello V, De Laurentiis M, Rea D, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20:150.
Ren C, Sun K, Zhang Y, et al. Sodium-glucose cotransporter-2 inhibitor empagliflozin ameliorates sunitinib-induced cardiac dysfunction via regulation of AMPK-mTOR signaling pathway-mediated autophagy. Front Pharmacol. 2021;12:664181.
Wright EM, Ghezzi C, Loo DDF. Novel and unexpected functions of SGLTs. Physiology (Bethesda). 2017;32:435–43.
Papadopoli D, Uchenunu O, Palia R, et al. Perturbations of cancer cell metabolism by the antidiabetic drug canagliflozin. Neoplasia. 2021;23:391–9.
Das A, Durrant D, Salloum FN, Xi L, Kukreja RC. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther. 2015;147:12–21.
Karami-Tehrani F, Moeinifard M, Aghaei M, Atri M. Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch Med Res. 2012;43:470–5.
Booth L, Roberts JL, Cruickshanks N, et al. PDE5 inhibitors enhance celecoxib killing in multiple tumor types. J Cell Physiol. 2015;230:1115–27.
Das A, Durrant D, Mitchell C, et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci U S A. 2010;107:18202–7.
Hamilton TK, Hu N, Kolomitro K, et al. Potential therapeutic applications of phosphodiesterase inhibition in prostate cancer. World J Urol. 2013;31:325–30.
Kim JH, Kim WS, Park C. Sildenafil prevents HDACi-induced Epstein-Barr virus reactivation through the PKG pathway in NK/T cell lymphoma; potential implications for HDACi-mediated fatal complications. Antiviral Res. 2021;189:105063.
Chelko SP, Keceli G, Carpi A, et al. Exercise triggers CAPN1-mediated AIF truncation, inducing myocyte cell death in arrhythmogenic cardiomyopathy. Sci Transl Med. 2021;13.
Wang Y, Zheng D, Wei M, et al. Over-expression of calpastatin aggravates cardiotoxicity induced by doxorubicin. Cardiovasc Res. 2013;98:381–90.
Wheeler HE, Maitland ML, Dolan ME, Cox NJ, Ratain MJ. Cancer pharmacogenomics: strategies and challenges. Nat Rev Genet. 2013;14:23–34.
Ramos KN, Gregornik D, Ramos KS. Pharmacogenomics insights into precision pediatric oncology. Curr Opin Pediatr. 2021;33:564–9.
Funding
This work was supported by the National Science Fund for Distinguished Young Scholars (Grant No. 82125004).
Author information
Authors and Affiliations
Contributions
Yiqi Zhao: literature searching and writing original draft. Hao Jia and Xiumeng Hua: reviewing the draft and editing. Jiangping Song: supervision and funding.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Jia, H., Hua, X. et al. Cardio-oncology: Shared Genetic, Metabolic, and Pharmacologic Mechanism. Curr Cardiol Rep 25, 863–878 (2023). https://doi.org/10.1007/s11886-023-01906-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-023-01906-6