Abstract
Purpose of Review
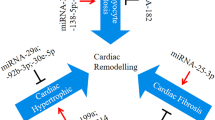
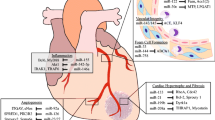
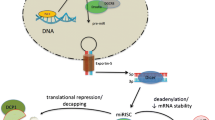
Heart failure is a severe clinical syndrome with complex and unclarified mechanisms, and it poses a serious threat to human health. MicroRNA, a non-coding RNA, can directly bind to target genes and regulate their expression. The important role of microRNAs in the development of HF has become a hot topic of research in recent years. This paper summarizes and prospects the mechanisms of microRNAs in regulating cardiac remodeling during heart failure to provide reference ideas for further research and clinical treatment.
Recent Findings
With extensive research, more target genes for microRNAs have been clarified. By modulating various molecules, microRNAs affect the contractile function of the myocardium and alter the process of myocardial hypertrophy, myocyte loss, and fibrosis, thereby interfering with the process of cardiac remodeling and exerting an important effect in the process of heart failure. Based on the above mechanism, microRNAs have promising applications in the diagnosis and treatment of heart failure.
Summary
MicroRNAs form a complex post-transcriptional control mechanism of gene expression, and the increase or decrease of their content during heart failure largely alters the course of cardiac remodeling. By continuously identifying their target genes, it is expected to achieve more precise diagnosis and treatment of this important topic of heart failure.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Luscher TF. Heart failure: the cardiovascular epidemic of the 21st century. Eur Heart J. 2015;36(7):395–7.
Ponikowski P, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25.
Azevedo PS, et al. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol. 2016;106(1):62–9.
Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35(3):569–82.
Konstam MA, et al. Left Ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC: Cardiovasc Imaging. 2011;4(1):98–108.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. (Windsor Mill, Md.). 2011;3(3):83–92.
Shivdasani RA. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006;108(12):3646–53.
Ovchinnikova ES, et al. Signature of circulating microRNAs in patients with acute heart failure. Eur J Heart Fail. 2016;18(4):414–23.
Sygitowicz G, Maciejak-Jastrzebska A, Sitkiewicz D. MicroRNAs in the development of left ventricular remodeling and postmyocardial infarction heart failure. Pol Arch Intern Med. 2020;130(1):59–65.
Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–49.
Bernardo BC, et al. Silencing of miR-34a attenuates cardiac dysfunction in a setting of moderate, but not severe, hypertrophic cardiomyopathy. PLoS ONE. 2014;9(2):e90337–e90337.
Hu J, et al. RBFox2-miR-34a-Jph2 axis contributes to cardiac decompensation during heart failure. Proc Natl Acad Sci. 2019;116(13):6172–80.
Sipido KR, et al. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc Res. 2002;53(4):782–805.
Stølen TO, et al. Exercise training reveals micro-RNAs associated with improved cardiac function and electrophysiology in rats with heart failure after myocardial infarction. J Mol Cell Cardiol. 2020;148:106–19.
• Younis NN, et al. Pachymic acid attenuated doxorubicin-induced heart failure by suppressing miR-24 and preserving cardiac junctophilin-2 in rats. Int J Mol Sci. 2021;22(19): p. 10710. This paper showed the possibility of using PA alone or as an adjuvant therapy with LN to attain better management of HF patients, especially those who developed tolerance toward LN.
Santulli G, Iaccarino G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas. 2016;93:65–72.
Bao M, et al. Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. Int J Mol Sci. 2013;14(11):23086–102.
Du Y, et al. Let-7a regulates expression of β1-adrenoceptors and forms a negative feedback circuit with the β1-adrenoceptor signaling pathway in chronic ischemic heart failure. Oncotarget. 2017;8(5):8752–64.
Liu Z, et al. Over-expression of microRNA-145 drives alterations in β-adrenergic signaling and attenuates cardiac remodeling in heart failure post myocardial infarction. Aging. 2020;12(12):11603–22.
•• Kwon J, et al. In Vivo Stimulation of α- and β-adrenoceptors in mice differentially alters small RNA content of circulating extracellular vesicles. Cells. 2021;10(5):1211. Findings from this study provided novel insight into chronic sympathetic nervous system activation in HF where excessive catecholamines may not only participate in pathological remodeling of the heart but also alter other organs due to secretion of EVs with altered miR content.
Chiong M, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2: e244.
Kansakar U, et al. Functional role of microRNAs in regulating cardiomyocyte death. Cells (Basel, Switzerland). 2022;11(6):983.
Sun S, Wang C, Weng J. MicroRNA‑138‑5p drives the progression of heart failure via inhibiting sirtuin 1 signaling. Mol Med Rep. 2021;23(4).
Lin B, et al. Sirt1 improves heart failure through modulating the NF-κB p65/microRNA-155/BNDF signaling cascade. Aging (Albany, NY.). 2021;13(10):14482–14498.
Li J, et al. ETS2 and microRNA-155 regulate the pathogenesis of heart failure through targeting and regulating GPR18 expression. Exp Ther Med. 2020;19(6):3469–78.
Wang Y, Zhang Y. LncRNA CAIF suppresses LPS-induced inflammation and apoptosis of cardiomyocytes through regulating miR-16 demethylation. Immunity, Inflammation and Disease. 2021;9(4):1468–78.
Yan K, et al. Mitochondrial miR-762 regulates apoptosis and myocardial infarction by impairing ND2. Cell Death Dis. 2019;10(7):500.
Chen C, et al. Differentially expressed lnc-NOS2P3-miR-939-5p axis in chronic heart failure inhibits myocardial and endothelial cells apoptosis via iNOS/TNFalpha pathway. J Cell Mol Med. 2020;24(19):11381–96.
Shi Y, et al. Cardiac-specific overexpression of miR-122 induces mitochondria-dependent cardiomyocyte apoptosis and promotes heart failure by inhibiting Hand2. J Cell Mol Med. 2021;25(11):5326–34.
Zhang Z, et al. Knockdown of MicroRNA-122 Protects H9c2 Cardiomyocytes from hypoxia-induced apoptosis and promotes autophagy. Med Sci Monit. 2017;23:4284–90.
Wang Y, et al. microRNA-454-mediated NEDD4-2/TrkA/cAMP axis in heart failure: Mechanisms and cardioprotective implications. J Cell Mol Med. 2021;25(11):5082–98.
Zhang B, et al. MiR-125b inhibits cardiomyocyte apoptosis by targeting BAK1 in heart failure. Mol Med. (Cambridge, Mass.). 2021:27(1):72–72.
Sun F, et al. LncRNA KCNQ1OT1 attenuates sepsis-induced myocardial injury via regulating miR-192–5p/XIAP axis. Exp Biol Med. (Maywood, N.J.). 2020;245(7):620–630.
Chen K, Zhang B, Sun Z. MicroRNA 379 regulates Klotho deficiency-induced cardiomyocyte apoptosis via repression of Smurf1. Hypertension. 2021;78(2):342–52.
Chun Y, Kim J. Autophagy: an essential degradation program for cellular homeostasis and life. Cells (Basel, Switzerland). 2018;7(12):278.
Galluzzi L, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36(13):1811–36.
Qi H, et al. MSTN attenuates cardiac hypertrophy through inhibition of excessive cardiac autophagy by blocking AMPK /mTOR and miR-128/PPARgamma/NF-kappaB. Mol Ther Nucleic Acids. 2020;19:507–22.
Zhan H, et al. Downregulation of miR-128 ameliorates Ang II-induced cardiac remodeling via SIRT1/PIK3R1 multiple targets. Oxid Med Cell Longev. 2021;2021:8889195–217.
Jin L, et al. MicroRNA302–367-PI3K-PTEN-AKT-mTORC1 pathway promotes the development of cardiac hypertrophy through controlling autophagy. In vitro cellular & developmental biology. Animal. 2019;56(2):112–119.
Xu X, et al. MicroRNA-17-5p Promotes cardiac hypertrophy by targeting Mfn2 to inhibit autophagy. Cardiovasc Toxicol. 2021;21(9):759–71.
Zhang Y, et al. MicroRNA-34c-5p provokes isoprenaline-induced cardiac hypertrophy by modulating autophagy via targeting ATG4B. Acta Pharm Sin B. 2022;12(5):2374–90.
Wu K, et al. MicroRNA-520d-3p alleviates hypoxia/reoxygenation-induced damage in human cardiomyocytes by targeting ATG-12. J Thromb Thrombolysis. 2021;52(2):429–39.
Chen YQ, et al. Knockdown of lncRNA TTTY15 alleviates myocardial ischemia-reperfusion injury through the miR-374a-5p/FOXO1 axis. IUBMB Life. 2021;73(1):273–85.
Zhang J, et al. Programmed necrosis in cardiomyocytes: mitochondria, death receptors and beyond. Br J Pharmacol. 2019;176(22):4319–39.
Liang Y, et al. Silenced SOX2-OT alleviates ventricular arrhythmia associated with heart failure by inhibiting NLRP3 expression via regulating miR-2355-3p. Immun Inflamm Dis. 2021;9(1):255–64.
Wang K, et al. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 2016;23(8):1394–405.
Wang JX, et al. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circ Res. 2015;117(4):352–63.
Bernardo BC, et al. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128(1):191–227.
Wehbe N, et al. MicroRNAs in cardiac hypertrophy. Int J Mol Sci. 2019;20(19):4714.
Bei Y, et al. Extracellular vesicles in cardiovascular theranostics. Theranostics. 2017;7(17):4168–82.
Tian C, et al. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am J Physiol Heart Circ Physiol. 2018;314(5):H928–39.
Tian C, et al. Extracellular vesicular MicroRNA-27a contributes to cardiac hypertrophy in chronic heart failure. J Mol Cell Cardiol. 2020;143:120–31.
Wang T, et al. NFATc3-dependent expression of miR-153-3p promotes mitochondrial fragmentation in cardiac hypertrophy by impairing mitofusin-1 expression. Theranostics. 2020;10(2):553–66.
Guo H, et al. mir15a/mir16‐1 cluster and its novel targeting molecules negatively regulate cardiac hypertrophy. Clin Transl Med. 2020;10(8):e242–n/a.
Asensio-Lopez MC, et al. The miRNA199a/SIRT1/P300/Yy1/sST2 signaling axis regulates adverse cardiac remodeling following MI. Sci Rep. 2021;11(1):3915.
Yan H, et al. Adeno-associated virus-mediated delivery of anti-miR-199a tough decoys attenuates cardiac hypertrophy by targeting PGC-1alpha. Mol Ther Nucleic Acids. 2021;23:406–17.
Li K, Lin Y, Li C. MiR-338-5p ameliorates pathological cardiac hypertrophy by targeting CAMKIIdelta. Arch Pharm Res. 2019;42(12):1071–80.
Fan P, et al. MiR-590-5p inhibits pathological hypertrophy mediated heart failure by targeting RTN4. J Mol Histol. 2021;52(5):955–64.
Chen H, Cai K. DSCAM-AS1 mediates pro-hypertrophy role of GRK2 in cardiac hypertrophy aggravation via absorbing miR-188–5p. In vitro cellular & developmental biology. Animal. 2020;56(4):286–295.
Long Y, Wang L, Li Z. SP1-induced SNHG14 aggravates hypertrophic response in in vitro model of cardiac hypertrophy via up-regulation of PCDH17. J Cell Mol Med. 2020;24(13):7115–26.
Yang X, et al. Long Noncoding RNA FTX reduces hypertrophy of neonatal mouse cardiac myocytes and regulates the PTEN/PI3K/Akt signaling pathway by sponging MicroRNA-22. Med Sci Monit. 2019;25:9609–17.
Zhang M, et al. Long non-coding RNA cardiac hypertrophy-associated regulator governs cardiac hypertrophy via regulating miR-20b and the downstream PTEN/AKT pathway. J Cell Mol Med. 2019;23(11):7685–98.
Frangogiannis NG. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99.
Creemers EE, van Rooij E. Function and therapeutic potential of noncoding rnas in cardiac fibrosis. Circ Res. 2016;118(1):108–18.
Zhang X, et al. The microRNA in ventricular remodeling: the miR-30 family. Biosci Rep. 2019;39(8):1.
Wang N, et al. Renal sympathetic denervation alleviates myocardial fibrosis following isoproterenol-induced heart failure. Mol Med Rep. 2017;16(4):5091–8.
Xu J, et al. MicroRNA-30c suppresses the pro-fibrogenic effects of cardiac fibroblasts induced byTGF-β1 and prevents atrial fibrosis by targeting TGF βRII. J Cell Mol Med. 2018;22(6):3045–57.
Li J, et al. Mir-30d regulates cardiac remodeling by intracellular and paracrine signaling. Circ Res. 2021;128(1):e1–23.
Pu L, et al. Exosomes released from mesenchymal stem cells overexpressing microRNA-30e ameliorate heart failure in rats with myocardial infarction. American journal of translational research. 2021;13(5):4007–25.
Yuan J, et al. Mir-21 Promotes cardiac fibrosis after myocardial infarction via targeting smad7. Cell Physiol Biochem. 2017;42(6):2207–19.
Zhou XL, et al. miR-21 promotes cardiac fibroblast-to-myofibroblast transformation and myocardial fibrosis by targeting Jagged1. J Cell Mol Med. 2018;22(8):3816–24.
Li D, et al. MicroRNA-21 mediates a positive feedback on angiotensin II-induced myofibroblast transformation. J Inflamm Res. 2020;13:1007–20.
Cao W, Shi P, Ge J. miR-21 enhances cardiac fibrotic remodeling and fibroblast proliferation via CADM1/STAT3 pathway. BMC Cardiovasc Disord. 2017;17(1):88–88.
Ramanujam D, et al. MicroRNA-21-dependent macrophage-to-fibroblast signaling determines the cardiac response to pressure overload. Circulation. 2021;143(15):1513–25.
Verjans R, et al. MicroRNA-221/222 family counteracts myocardial fibrosis in pressure overload–induced heart failure. Hypertension. 2018;71(2):280–8.
Zhou Y, Richards AM, Wang P. MicroRNA-221 is cardioprotective and anti-fibrotic in a rat model of myocardial infarction. Molecular therapy Nucleic acids. 2019;17:185–97.
Zhou Y, et al. microRNA-221 inhibits latent TGF-β1 activation through targeting thrombospondin-1 to attenuate kidney failure-induced cardiac fibrosis. Molecular therapy Nucleic acids. 2020;22:803–14.
Wang Q, et al. miR‑320a in serum exosomes promotes myocardial fibroblast proliferation via regulating the PIK3CA/Akt/mTOR signaling pathway in HEH2 cells. Exp Ther Med. 2021;22(2).
Li F, et al. miR-320 accelerates chronic heart failure with cardiac fibrosis through activation of the IL6/STAT3 axis. Aging (Albany NY). 2021;13(18):22516–27.
Zhang X, et al. The double face of miR-320: cardiomyocytes-derived miR-320 deteriorated while fibroblasts-derived miR-320 protected against heart failure induced by transverse aortic constriction. Signal Transduct Target Ther. 2021;6(1):69–69.
Vegter EL, et al. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail. 2016;18(5):457–68.
Poller W, et al. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J. 2018;39(29):2704–16.
Kennel PJ, Schulze PC. A review on the evolving roles of MiRNA-based technologies in diagnosing and treating heart failure. Cells. 2021;10(11).
Halushka PV, Goodwin AJ, Halushka MK. Opportunities for microRNAs in the crowded field of cardiovascular biomarkers. Annu Rev Pathol. 2019;14:211–38.
Chen Y, et al. Heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF): the diagnostic value of circulating microRNAs. Cells (Basel, Switzerland). 2019;8(12):1651.
Su Y, et al. Circulating miR-19b-3p as a novel prognostic biomarker for acute heart failure. J Am Heart Assoc. 2021;10(20): e022304.
Ding H, et al. Combined detection of miR-21–5p, miR-30a-3p, miR-30a-5p, miR-155–5p, miR-216a and miR-217 for screening of early heart failure diseases. Biosci Rep. 2020;40(3).
Taubel J, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J. 2021;42(2):178–88.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31971046).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, YT., Xiao, YC., Xu, Yl. et al. The Effects of MicroRNAs in the Development of Heart Failure. Curr Cardiol Rep 25, 747–759 (2023). https://doi.org/10.1007/s11886-023-01895-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-023-01895-6