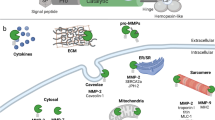
Abstract
Purpose of Review
Genome-wide association studies have repeatedly linked the metalloproteinase ADAMTS7 to coronary artery disease. Here we aim to highlight recent findings surrounding the human genetics of ADAMTS7, novel mouse models that investigate ADAMTS7 function, and potential substrates of ADAMTS7 cleavage.
Recent Findings
Recent genome-wide association studies in coronary artery disease have replicated the GWAS signal for ADAMTS7 and shown that the signal holds true even across different ethnic groups. However, the direction of effect in humans remains unclear. A recent novel mouse model revealed that the proatherogenicity of ADAMTS7 is derived from its catalytic functions, while at the translational level, vaccinating mice against ADAMTS7 reduced atherosclerosis. Finally, in vitro proteomics approaches have identified extracellular matrix proteins as candidate substrates that may be causal for the proatherogenicity of ADAMTS7.
Summary
ADAMTS7 represents an enticing target for therapeutic intervention. The recent studies highlighted here have replicated prior findings, confirming the genetic link between ADAMTS7 and atherosclerosis, while providing further evidence in mice that ADAMTS7 is a targetable proatherogenic enzyme.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023. https://doi.org/10.1161/CIR.0000000000001123.
Aragam KG, Jiang T, Goel A, Kanoni S, Wolford BN, Atri DS, et al. Discovery and systematic characterization of risk variants and genes for coronary artery disease in over a million participants. Nat Genet. 2022;54(12):1803–15. https://doi.org/10.1038/s41588-022-01233-6.
• Tcheandjieu C, Zhu X, Hilliard AT, Clarke SL, Napolioni V, Ma S, et al. Large-scale genome-wide association study of coronary artery disease in genetically diverse populations. Nat Med. 2022;28(8):1679–92. https://doi.org/10.1038/s41591-022-01891-3. This GWAS revealed that ADAMTS7 reached genome wide significance in participants of Hispanic descent.
Coronary Artery Disease Genetics C. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43(4):339–44. https://doi.org/10.1038/ng.782.
Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121–30. https://doi.org/10.1038/ng.3396.
Erdmann J, Kessler T, Munoz Venegas L, Schunkert H. A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovasc Res. 2018;114(9):1241–57. https://doi.org/10.1093/cvr/cvy084.
Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. https://doi.org/10.1016/S0140-6736(13)61752-3.
Libby P, Hansson GK. From focal lipid storage to systemic inflammation: JACC review topic of the week. J Am Coll Cardiol. 2019;74(12):1594–607. https://doi.org/10.1016/j.jacc.2019.07.061.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914.
Reilly MP, Li M, He J, Ferguson JF, Stylianou IM, Mehta NN, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377(9763):383–92. https://doi.org/10.1016/S0140-6736(10)61996-4.
Lu X, Wang L, Chen S, He L, Yang X, Shi Y, et al. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat Genet. 2012;44(8):890–4. https://doi.org/10.1038/ng.2337.
Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–8. https://doi.org/10.1038/ng.784.
van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. 2018;122(3):433–43. https://doi.org/10.1161/CIRCRESAHA.117.312086.
Liu CJ, Kong W, Ilalov K, Yu S, Xu K, Prazak L, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J. 2006;20(7):988–90. https://doi.org/10.1096/fj.05-3877fje.
Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386(Pt 1):15–27. https://doi.org/10.1042/BJ20040424.
Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 2015;16(1):113. https://doi.org/10.1186/s13059-015-0676-3.
Plaimauer B, Zimmermann K, Volkel D, Antoine G, Kerschbaumer R, Jenab P, et al. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13). Blood. 2002;100(10):3626–32. https://doi.org/10.1182/blood-2002-05-1397.
Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211–25. https://doi.org/10.1146/annurev-med-061813-013241.
Wiernek SL, Jiang B, Gustafson GM, Dai X. Cardiac implications of thrombotic thrombocytopenic purpura. World J Cardiol. 2018;10(12):254–66. https://doi.org/10.4330/wjc.v10.i12.254.
Tan Ide A, Ricciardelli C, Russell DL. The metalloproteinase ADAMTS1: a comprehensive review of its role in tumorigenic and metastatic pathways. Int J Cancer. 2013;133(10):2263–76. https://doi.org/10.1002/ijc.28127.
Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5(8):927–46.
Camejo G, Hurt-Camejo E, Wiklund O, Bondjers G. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 1998;139(2):205–22. https://doi.org/10.1016/s0021-9150(98)00107-5.
Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15(7):483–94. https://doi.org/10.1038/sj.cr.7290318.
Neufeld EB, Zadrozny LM, Phillips D, Aponte A, Yu ZX, Balaban RS. Decorin and biglycan retain LDL in disease-prone valvular and aortic subendothelial intimal matrix. Atherosclerosis. 2014;233(1):113–21. https://doi.org/10.1016/j.atherosclerosis.2013.12.038.
Didangelos A, Mayr U, Monaco C, Mayr M. Novel role of ADAMTS-5 protein in proteoglycan turnover and lipoprotein retention in atherosclerosis. J Biol Chem. 2012;287(23):19341–5. https://doi.org/10.1074/jbc.C112.350785.
Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–52. https://doi.org/10.1038/nature03417.
Wang Z, Ye D, Ye J, Wang M, Liu J, Jiang H, et al. ADAMTS-5 Decreases in coronary arteries and plasma from patients with coronary artery disease. Dis Markers. 2019;2019:6129748. https://doi.org/10.1155/2019/6129748.
Kumar S, Chen M, Li Y, Wong FH, Thiam CW, Hossain MZ, et al. Loss of ADAMTS4 reduces high fat diet-induced atherosclerosis and enhances plaque stability in ApoE(-/-) mice. Sci Rep. 2016;6:31130. https://doi.org/10.1038/srep31130.
Davis JP, Huyghe JR, Locke AE, Jackson AU, Sim X, Stringham HM, et al. Common, low-frequency, and rare genetic variants associated with lipoprotein subclasses and triglyceride measures in Finnish men from the METSIM study. PLoS Genet. 2017;13(10):e1007079. https://doi.org/10.1371/journal.pgen.1007079.
Graham SE, Clarke SL, Wu KH, Kanoni S, Zajac GJM, Ramdas S, et al. The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600(7890):675–9. https://doi.org/10.1038/s41586-021-04064-3.
Wang L, Zheng J, Bai X, Liu B, Liu CJ, Xu Q, et al. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. 2009;104(5):688–98. https://doi.org/10.1161/CIRCRESAHA.108.188425.
Bauer RC, Tohyama J, Cui J, Cheng L, Yang J, Zhang X, et al. Knockout of Adamts7, a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation. 2015;131(13):1202–13. https://doi.org/10.1161/CIRCULATIONAHA.114.012669.
Kathiresan S, Manning AK, Demissie S, D’Agostino RB, Surti A, Guiducci C, et al. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S17. https://doi.org/10.1186/1471-2350-8-S1-S17.
Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, et al. Genetics of blood lipids among ~300,000 multi-ethnic participants of the Million Veteran Program. Nat Genet. 2018;50(11):1514–23. https://doi.org/10.1038/s41588-018-0222-9.
Pu X, Xiao Q, Kiechl S, Chan K, Ng FL, Gor S, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet. 2013;92(3):366–74. https://doi.org/10.1016/j.ajhg.2013.01.012.
Consortium CAD, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45(1):25–33. https://doi.org/10.1038/ng.2480.
Boughton AP, Welch RP, Flickinger M, VandeHaar P, Taliun D, Abecasis GR, et al. LocusZoom.js: interactive and embeddable visualization of genetic association study results. Bioinformatics. 2021;37(18):3017–8. https://doi.org/10.1093/bioinformatics/btab186.
Kim-Hellmuth S, Aguet F, Oliva M, Munoz-Aguirre M, Kasela S, Wucher V, et al. Cell type-specific genetic regulation of gene expression across human tissues. Science. 2020;369(6509). https://doi.org/10.1126/science.aaz8528.
Cheetham SW, Faulkner GJ, Dinger ME. Overcoming challenges and dogmas to understand the functions of pseudogenes. Nat Rev Genet. 2020;21(3):191–201. https://doi.org/10.1038/s41576-019-0196-1.
Tominaga K, Kirtane B, Jackson JG, Ikeno Y, Ikeda T, Hawks C, et al. MRG15 regulates embryonic development and cell proliferation. Mol Cell Biol. 2005;25(8):2924–37. https://doi.org/10.1128/MCB.25.8.2924-2937.2005.
Wei Y, Tian C, Zhao Y, Liu X, Liu F, Li S, et al. MRG15 orchestrates rhythmic epigenomic remodelling and controls hepatic lipid metabolism. Nat Metab. 2020;2(5):447–60. https://doi.org/10.1038/s42255-020-0203-z.
Saleheen D, Zhao W, Young R, Nelson CP, Ho W, Ferguson JF, et al. Loss of cardioprotective effects at the aDAMTS7 locus as a result of gene-smoking interactions. Circulation. 2017;135(24):2336–53. https://doi.org/10.1161/CIRCULATIONAHA.116.022069.
• Matsunaga H, Ito K, Akiyama M, Takahashi A, Koyama S, Nomura S, et al. Transethnic meta-analysis of genome-wide association studies identifies three new loci and characterizes population-specific differences for coronary artery disease. Circ Genom Precis Med. 2020;13(3):e002670. https://doi.org/10.1161/CIRCGEN.119.002670. This GWAS revealed that ADAMTS7 reached genome wide significance in participants of Japanese descent.
Pasterkamp G, van der Laan SW, Haitjema S, Foroughi Asl H, Siemelink MA, Bezemer T, et al. Human validation of genes associated with a murine atherosclerotic phenotype. Arterioscler Thromb Vasc Biol. 2016;36(6):1240–6. https://doi.org/10.1161/ATVBAHA.115.306958.
•• Mizoguchi T, MacDonald BT, Bhandary B, Popp NR, Laprise D, Arduini A, et al. Coronary disease association with ADAMTS7 is due to protease activity. Circ Res. 2021;129(4):458–70. https://doi.org/10.1161/CIRCRESAHA.121.319163. This publication revealed that the proatherogenecity of ADAMTS7 observed in mice is due to its catayltic activity.
•• Ma Z, Mao C, Chen X, Yang S, Qiu Z, Yu B, et al. Peptide vaccine against ADAMTS-7 ameliorates atherosclerosis and postinjury neointima hyperplasia. Circulation. 2022; https://doi.org/10.1161/CIRCULATIONAHA.122.061516. This publication showed that therapeutic intervention at the ADAMTS7 level can reduce atherosclerosis in mice.
Kessler T, Zhang L, Liu Z, Yin X, Huang Y, Wang Y, et al. ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation. 2015;131(13):1191–201. https://doi.org/10.1161/CIRCULATIONAHA.114.014072.
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56. https://doi.org/10.1038/s41572-019-0106-z.
• Colige A, Monseur C, Crawley JTB, Santamaria S, de Groot R. Proteomic discovery of substrates of the cardiovascular protease ADAMTS7. J Biol Chem. 2019;294(20):8037–45. https://doi.org/10.1074/jbc.RA119.007492. This publication was the first to employ TAILS in ADAMTS7 and revealed the ECM as the most likely site of ADAMTS7 action.
• MacDonald BT, Keshishian H, Mundorff CC, Arduini A, Lai D, Bendinelli K, et al. TAILS identifies candidate substrates and biomarkers of ADAMTS7, a therapeutic protease target in coronary artery disease. Mol Cell Proteomics. 2022;21(4):100223. https://doi.org/10.1016/j.mcpro.2022.100223. This publication performed TAILS in SMCs and endothelial cells and expanded on the list of candidate substrates of ADAMTS7.
Kleifeld O, Doucet A, Prudova A, Auf dem Keller U, Gioia M, Kizhakkedathu JN, et al. Identifying and quantifying proteolytic events and the natural N terminome by terminal amine isotopic labeling of substrates. Nat Protoc. 2011;6(10):1578–611. https://doi.org/10.1038/nprot.2011.382.
Su CT, Urban Z. LTBP4 in health and disease. Genes (Basel). 2021;12(6). https://doi.org/10.3390/genes12060795.
Toma I, McCaffrey TA. Transforming growth factor-beta and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012;347(1):155–75. https://doi.org/10.1007/s00441-011-1189-3.
Djokic J, Fagotto-Kaufmann C, Bartels R, Nelea V, Reinhardt DP. Fibulin-3, -4, and -5 are highly susceptible to proteolysis, interact with cells and heparin, and form multimers. J Biol Chem. 2013;288(31):22821–35. https://doi.org/10.1074/jbc.M112.439158.
Bekhouche M, Leduc C, Dupont L, Janssen L, Delolme F, Vadon-Le Goff S, et al. Determination of the substrate repertoire of ADAMTS2, 3, and 14 significantly broadens their functions and identifies extracellular matrix organization and TGF-beta signaling as primary targets. FASEB J. 2016;30(5):1741–56. https://doi.org/10.1096/fj.15-279869.
Duband JL, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55(1):1–11. https://doi.org/10.1111/j.1432-0436.1993.tb00027.x.
Funding
This work was supported by an American Heart Association predoctoral fellowship to A.C. (909206), grants from the National Institutes of Health to M.P.R. (R01HL150359, R01HL166916, and UL1TR001873), a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute to R.C.B (R01HL141745), and institutional funds from Columbia University to R.C.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have nothing to disclose.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chung, A., Reilly, M.P. & Bauer, R.C. ADAMTS7: a Novel Therapeutic Target in Atherosclerosis. Curr Atheroscler Rep 25, 447–455 (2023). https://doi.org/10.1007/s11883-023-01115-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-023-01115-0